Reviewed By Allergy, Immunology & Inflammation Assembly
Submitted by
P. Scott Meehan MD
Fellow
Division of Pulmonary, Allergy, Critical Care and Sleep Medicine
The Ohio State University Medical Center
Columbus, Ohio
Jennifer W. McCallister MD
Assistant Professor of Internal Medicine
Division of Pulmonary, Allergy, Critical Care and Sleep Medicine
The Ohio State University Medical Center
Columbus, Ohio
Submit your comments to the author(s).
History
A 27 year old Caucasian woman with a history of uncontrolled asthma, allergic rhinitis, gastroesophageal reflux disease and recurrent pneumonias was referred for evaluation in the pulmonary clinic. She was diagnosed with asthma at age twelve. In the last six years she had been treated as an outpatient for pneumonia, sinusitis, and bronchitis several times per year and had required systemic corticosteroids several times per year for presumed asthma exacerbations.
Since last year she described persistent dyspnea with exertion, daily cough, and thick, yellow sputum production which had worsened. This had not improved despite multiple courses of antibiotics, systemic glucocorticoids, and escalation of her inhaled corticosteroids. She also reported wheezing and recurrent episodes of left sided chest pain. There was no nighttime cough or limitation in daily activities. She had not had frequent ear infections, yeast infections, epistaxis or hearing loss.
Her medications included a combination high dose inhaled corticosteroid and long acting beta agonist, montelukast, a proton pump inhibitor, albuterol, a multi-vitamin and methylphenidate. She had been using short acting beta agonists multiple times per day without relief.
She had a 5 pack-year smoking history and quit eight years prior. She was married and had been trying to conceive for about 15 months without success. She had no known occupational exposures and owned one dog. There was no known family history of asthma or other respiratory diseases.
Physical Exam
On physical exam, she was a well developed, obese (Body Mass Index 32 kg/m2) woman in no acute distress. Her resting oxygen saturation (SpO2) was 97% on room air. Head and neck exam were normal. Heart sounds were normal without murmur, rub, or gallop with sounds heard best at right sternal border. Auscultation of the lungs revealed inspiratory squeaks over the left upper lung field, otherwise clear. Percussion and diaphragmatic excursion were normal. The abdomen was soft and non-tender with no hepatosplenomegaly appreciated. There was no peripheral edema or clubbing.
Laboratory Data
| White blood cells | 6.2 K/uL |
| Hemoglobin | 12.4 g/dL |
| Hematocrit | 39.4 % |
| Platelets | 285 K/uL |
| Differential, normal | |
| Alpha 1 Anti-trypsin | 117 mg/dL (80-240 mg/dL) |
| Immunoglobulin G (IgG) | 1001 mg/dL (700-1600 mg/dL) |
| Immunoglobulin A | 312 mg/dL (70-400 mg/dL) |
| Immunoglobulin M | 116 mg/dL (40-230 mg/dL) |
| Immunoglobulin E | 18.4 IU/mL (7-135 IU/mL) |
| Total hemolytic complement titer (CH50) | 115 units (51-150 units) |
| Pneumococcal antibody titer normal | |
| Tetanus IgG antibody titer normal | |
| Diphtheria IgG antibody titer normal |
Pulmonary Function Testing
| Forced Vital Capacity (FVC) | 3.54 L (109% predicted) |
| Forced expiratory volume in 1 second (FEV1) | 2.94 L (105% predicted) |
| FEV1/FVC | 81.4% |
| Total Lung Capacity (TLC) | 4.5 L (104% predicted) |
| Diffusion Capacity of Carbon Monoxide (DLCO) | 19 mL/min/mm Hg (82% predicted) |
| Flow volume loop normal |
Figures
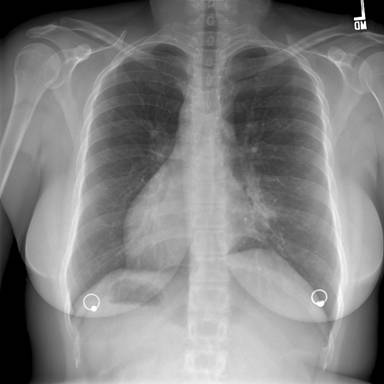
Figure 1: Posterior-Anterior Chest Radiograph—demonstrates dextrocardia and a gastric bubble under the right hemi-diaphragm consistent with situs inversus with a left sided infiltrate and tram tracking along the left heart border. Follow-up imaging with computed tomography (CT) of the chest, abdomen, and pelvis was obtained with selected images provided below:
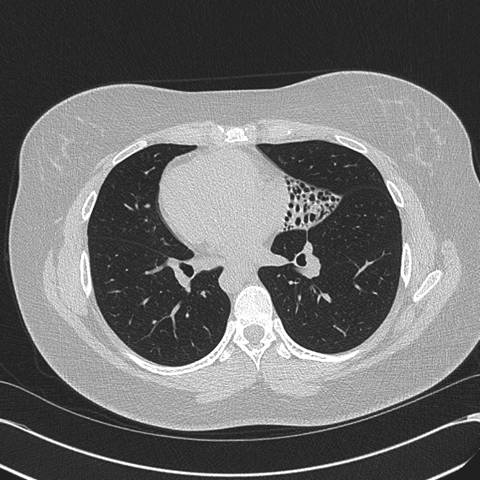
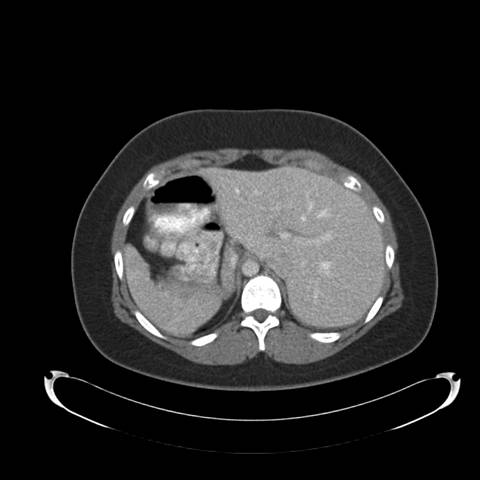
Figure 2: High resolution computed tomography (CT) of the Chest —demonstrates situs inversus totalis and volume loss of the "left middle lobe" with bronchiectasis, presumably fibrotic.
References
- Afzelius BA: A human syndrome caused by immotile cilia. Science 1976;193: 371-379.
- Noone PG, Bali D, Carson JL, Sannuti A, Gipson CL, Osrowski LE, Bromberg PA, Boucher RC, Knowles MR. Discordant organ laterality in momozygotic twins with primary ciliary dyskinesia. Am J Med Genet 1999;82:155-160.
- Leigh M, Pittman J, Carson J, Ferkol T, Dell S, Davis S, Knowles M, Zariwala, M. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genetics in Medicine 2009; 11(7):473-487.
- Barbato A, Frischer T, Keuhni CE, Azevedo S, Baktai G, Bartoloni L, Eber E, Escibano A, Haarman E, Hesselmar B, Hogg C, Jorrissen M, Lucas J, Nielsen KG, O’Callaghan C, Omran H, Pohunek P, Strippoli M-PF, Bush A. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. European Respiratory Journal 2009; 34:1264-1276.
- Meeks, M. and Bush, A. State of the Art, Primary Ciliary Dyskinesia (PCD). Pediatric Pulmonology 2000;29:307-316.
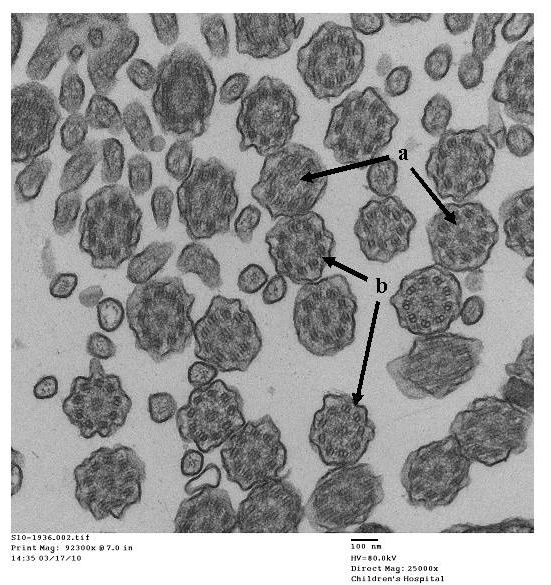
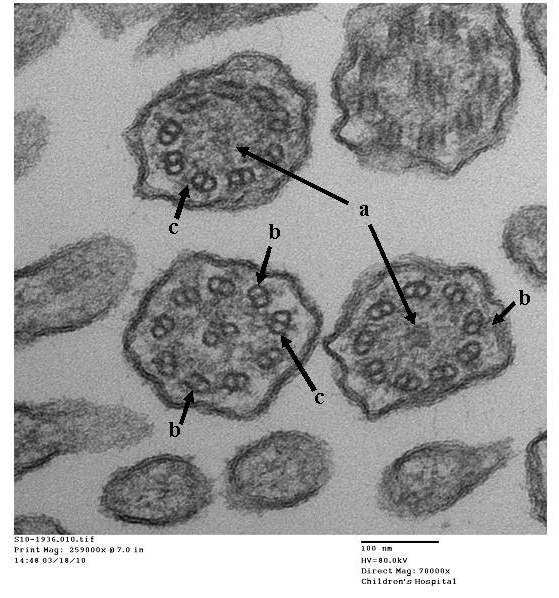
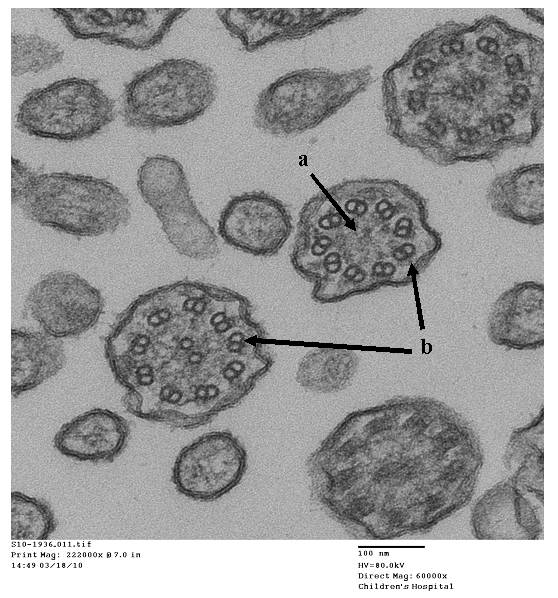
- Papon JF: A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. European Respiratory Journal 2010;35(5):1057-1063.
- Corbelli R, Bringolf-Isler B, Amacher A, Sasse B, Spycher M, Hammer J. Nasal nitric oxide measurements to screen children for primary ciliary dyskinesia. Chest 2004;126:1054-1059.
- Rosen, MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest 2006;129:122S-131S.
- Amirav I, Choehn-Cymberknoh M, Shoseyov D, Kerem E. Primary ciliary dyskinesia: prospects for new therapies, building on the experience in cystic fibrosis. Paediatr Respir Rev 2009;10:58-62. 129:122S-131S.
- Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med 2008;102:1494-1496.
- Elkins MR, Robinson M, Rose BR et al. National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic slaine in patients with cystic fibrosis. N Engl J Med 2006;354:229-240.
- Fauroux B, Tamalet A, Clement A. Management of primary ciliary dyskinesia: the lower airways. Paediatr Respir Rev 2009; 10: 55-57.
- Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J 1997;10:2376-2379.
- Marthin, JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Repsir Crit Care Med 2010;181:1262-1268.
- Munro NC, Currie DC, Lindsay KS. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. Thorax 1994;49:684-687. Blyth M. Ectopic pregnancy in primary cliliary dyskinesia. J Obstet Gynaecol 2008;28(3):358.
- Blyth M. Ectopic pregnancy in primary cliliary dyskinesia. J Obstet Gynaecol 2008;28(3):358.
- Kennedy MP, Omran H, Leight MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, Ahrens P, Lange L, Morillas HN, Noone PG, Zariwala MA, Knowles MR. Congenital heart disease and other heterotaxic defects in a large cohort of patient with primary ciliary dyskinesia. Circulation 2007;115(22):2814-2821.