Massive Pulmonary Embolism
Claire L. Keating, M.D.
Jennifer A. Cunningham, M.D.
Columbia University College of Physicians and Surgeons
HISTORY:
55-year-old female nursing home resident with past medical history of AIDS, dilated cardiomyopathy (estimated left ventricular ejection fraction 15% on a previous transthoracic echocardiogram), and prior deep venous thrombosis (DVT) was found to be hypotensive and in respiratory distress while at her skilled nursing facility.
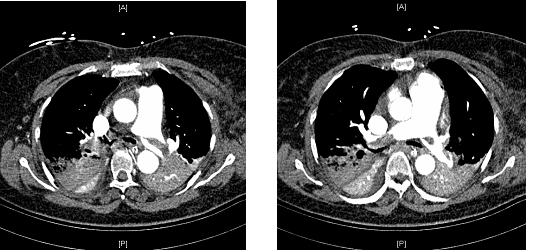
She was brought to the emergency department, where vital signs were notable for temperature of 100.9ºF, HR=142/min, BP=90/60 mmHg after intravenous fluids, with oxygen saturation of 99% while breathing 100% oxygen via non-rebreather mask. Computed tomography of the chest with pulmonary angiogram protocol (Figure 1) revealed large, thrombi in the right main and left main pulmonary arteries with incomplete occlusion, in addition to multiple segmental thrombi in right upper, middle and lower lobes. No lower extremity deep vein thromboses were noted on venogram. Anticoagulation was initiated and the patient was transferred to the intensive care unit (ICU) for further management.
FIGURE 1: CT scan of the chest demonstrating pulmonary emboli in bilateral main pulmonary artery
Question 1
What distinguishes massive from submassive pulmonary embolism?
- the presence of hypoxemia
- the presence of right ventricular dysfunction
- the presence of shock
- the presence of pulmonary hypertension
- the presence of concurrent deep venous thrombosis
Answer to Question 1
Correct answer: C
The main criteria defining a massive pulmonary embolism are signs of hemodynamic compromise [1]. These include:
-Arterial hypotension defined as systolic arterial blood pressure <90mmHg or a drop in systolic arterial blood pressure of at least 40mmHg for at least 15 minutes (mortality 15%)
-Cardiogenic shock as manifested by tissue hypoperfusion and hypoxia, altered level of consciousness, oliguria, or cool, clammy extremities (mortality 25%)
-Circulatory collapse requiring cardiopulmonary resuscitation (mortality 65%)
Patients with submassive pulmonary emboli are normotensive with signs of right ventricular dysfunction present (see below).
PAST MEDICAL HISTORY:
AIDS (CD4+ cell count=20/mm3)
Multiple cerebrovascular infarcts with residual expressive aphasia and hemiparesis
Dilated cardiomyopathy (presumed HIV-related)
Hypertension
Chronic kidney disease with baseline serum creatinine of 1.5 mg/dL
Past DVT (not on anticoagulation for unclear reasons)
MEDICATIONS:
Clopidogrel
ASA
Enalapril
Furosemide
Levetiracetam
Abacavir
Lamivudine
Zidovudine
Efavirenz
PHYSICAL EXAM:
Upon admission to ICU
Vitals: T=100.1ºF, HR=112/min, BP=91/63 mmHg, RR=28/min,
SpO2=96% on 100% oxygen via nonrebreather mask
General: awake, nonverbal, dyspneic and diaphoretic
HEENT: Eyes deviated left, pupils 3mm and reactive, JVP estimated at 8cm H2O
Heart: tachycardic, regular with frequent ectopy, grade 3/6 holosystolic murmur and S3 gallop present, point of maximal impulse displaced laterally
Lungs: coarse breath sounds bilaterally
Abdomen: soft, nontender, hypoactive bowel sounds, pulsatile liver, brown guaiac negative stool
Extremities: right upper extremity with decreased tone, 1+ edema in lower extremities bilaterally, all extremities cool to touch
Neurologic: withdrawal to pain in all extremities, spontaneous eye opening, non-attentive, nonverbal and not following commands.
ADMISSION LABORATORY VALUES:
White blood count 14,600/mm3
Hemoglobin 10.6 g/dL
Platelets 125,000/ mm3
Sodium 134 mmol/L
Potassium 4.6 mmol/L
Chloride 107 mmol/L
Bicarbonate 13 mmol/L
Blood urea nitrogen 45 mg/dL
Creatinine 2.8 mg/dL (baseline 1.5)
Serum glucose 102 mg/dL
Troponin 2.7 ng/mL (upper limit normal = 0.08)
BNP 1,944 pg/mL (upper limit normal = 100-400)
Arterial Blood Gases:
Emergency Room (on 100% oxygen via non-rebreather mask):
pH=7.32
PaCO2=25 mmHg
PaO2=250 mmHg
ICU (prior to intubation, on 100% oxygen via non-rebreather mask):
pH=7.04
PaCO2=38 mmHg
PaO2=71 mmHg
Question 2
What echocardiogram findings are seen in submassive and massive pulmonary embolism?
- right ventricular dilation
- right ventricular hypokinesis with sparing of the right ventricular apex (McConnell sign)
- loss of inspiratory collapse on inferior vena cava
- paradoxical septal wall motion
- all of the above
Answer to Question 2
Correct answer: E
Doppler echocardiogram can be useful in supporting the diagnosis of submassive and massive pulmonary embolism, especially in the cases where a contrast chest CT cannot be performed immediately. Findings on Doppler echocardiogram demonstrate acute right ventricular pressure overload in the absence of left ventricular or mitral valve disease with or without increased pulmonary artery pressures. These findings typically occur only after >30% of the pulmonary vascular cross-sectional area is impaired and include [2]:
- right ventricular dilatation (larger than the left ventricle from the apical or subcostal view) and hypertrophy (about 6 mm; normal <4mm)
- right ventricular hypokinesis with sparing of the right ventricular apex (McConnell sign)
- right pulmonary artery dilatation
- paradoxical septal wall motion (interventricular septum bulges towards the left ventricle)
- loss of inspiratory collapse of inferior vena cava
- elevated pulmonary artery systolic pressure as estimated by the gradient across the tricuspid valve
- small difference in LV area during diastole and systole (low cardiac output)
- patent foramen ovale
Question 3
What is the preferred hemodynamic support for hypotension in massive pulmonary embolism?
- intravenous fluids
- norepinephrine
- inotropic agents, such as isoproteronol
- vasopressin
- intra-aortic balloon counter-pulsation device (IABP)
Answer to Question 3
Correct answer: B
Norepinephrine is the preferred agent for hemodynamic support in massive pulmonary embolism with hypotension. This is based on several studies using canine models of pulmonary embolism [4-6], where isoproterenol or norepinephrine were administered for hemodynamic support in acute pulmonary embolism. Success in achieving hemodynamic stability and improvement in ventricular wall function was higher in dogs receiving infusions of norepinephrine. The effect is hypothesized to be due to increased systemic pressures, resulting in improved coronary perfusion and improved right ventricular function. In patients with less severe hypotension and more severe cardiac dysfunction, inotropic agents may be considered as an adjunct or alternative to norepinephrine [6-11]. Newer inotropic agents, such as amrinone, which act as both inotropic agent and pulmonary vasodilator have shown promise in animal studies and case reports [12,13].
A number of detrimental effects of intravenous fluids have been documented in animal studies, including decreased cardiac output and diminished right coronary artery blood flow due to increased right ventricular dilation [4-9]. In the face of diminished right coronary artery flow, worsening right ventricular ischemia can lead to diminished RV systolic function, establishing a vicious cycle of auto-aggravation. One study in humans [14], however, suggested that a 500 ml fluid load may initially improve cardiac output among patients with massive PE, although the long-term effects of fluid administration on cardiac function and hemodynamics are unclear. Most authors would agree that intravenous fluids must be used with caution in patients with massive PE [15-17].
HOSPITAL COURSE
After admission to the ICU, the patient received an intravenous infusion of unfractionated heparin drip and an intravenous infusion of norepinephrine at 5 micrograms/minute for hemodynamic support. A Foley catheter was placed with urine output remaining <0.5 mL/kg/hour despite a trial of intravenous fluid resuscitation. Bedside transthoracic echocardiogram was performed and demonstrated a dilated left ventricle with depressed systolic function with an estimated left ventricular ejection fraction of 15% (unchanged from baseline echo), in addition to a new finding of moderate right ventricular and right atrial dilatation with a calculated RV systolic pressure of 58mmHg (increased RV dysfunction from the prior study). There was moderate tricuspid regurgitation and a dilated inferior vena cava noted. Consideration was given to systemic thrombolysis due to the presence of persistent hypotension and end organ dysfunction, however, with a therapeutic partial thromboplastin time (PTT) on heparin, massive hemoptysis (>250 cc with >2g/dL hemoglobin drop) developed. The trachea was urgently intubated and heparin was discontinued. Interventional radiology was consulted for catheter thrombectomy and inferior vena caval (IVC) filter placement.
Question 4
In cases of massive pulmonary embolism, what options remain when systemic thrombolysis cannot be performed safely?
- surgical embolectomy
- catheter-directed thrombolysis
- percutaneous embolectomy
- percutaneous thrombus fragmentation
- all of the above
Answer to Question 4
Correct answer: E
Surgical embolectomy:
Surgical embolectomy involves transection of the main pulmonary artery via sternotomy incision with manual extraction of thromboembolism. Although in the past, peri-operative mortality was a high as 57%, some experienced centers now report peri-operative mortality of approximately 6% [33]. However, with the use of cardiopulmonary bypass and increasing surgical expertise, mortality and morbidity from surgical embolectomy can be minimized,[18,19] and may offer benefit particularly to those patients with evidence of pulmonary hypertension [18].
Historically, surgical embolectomy was the only available option for patients who fail or who have contraindications to systemic thrombolysis. It is not clear what role it will play in the future given the advent of other interventional options (listed below).
Catheter-directed thrombolysis:
This technique requires placement of an intra-arterial catheter to the site of the embolus with bolus and infusion of a thrombolytic agent [20]. Catheter-directed thrombolysis usually requires concurrent intravenous unfractionated heparin administration.
Small studies, including case series and controlled trials, have evaluated the efficacy of intrapulmonary thrombolysis [21-23]. Although clinical endpoints such as mortality were not evaluated, these studies suggest equivalent or superior radiographic resolution of thrombolysis compared to systemic thrombolysis. Bleeding complication rates were low following intrapulmonary thrombolysis, suggesting that catheter-directed thrombolysis may be possible even in patients who have contraindications to systemic thrombolysis [29]. It is noteworthy, however, that these regimens also utilized systemic anticoagulation. Therefore, caution must be exercised in extrapolating the results of these small studies to patients with contraindications to systemic thrombolysis or anticoagulation. Further investigation into the safety of this technique in high risk patient populations is needed.
Percutaneous aspiration thrombectomy or fragmentation:
When systemic or intrapulmonary thrombolysis and surgical embolectomy are not possible, there are a number of interventional options available that aim to rapidly relieve central obstruction and restore hemodynamic stability [20].
Greenfield embolectomy catheter [20]: This catheter (Boston Scientific/Meditech; Watertown, MA) is inserted into the site of the thrombus, and with manual suction using a large syringe retrieves the clot, which is then removed en bloc through the venotomy site or vascular sheath.
Rotatable pigtail catheter [20]: The pigtail tip of this catheter (Cook Europe; Bjaeverskov, Denmark) is rotated either by hand or by an attachable low-speed electric catheter to disrupt the intrapulmonary clot into smaller fragments which then migrate into the distal pulmonary circulation. The catheter can be advanced into peripheral pulmonary branches and manually rotated to further clot fragmentation.
Rheolytic thrombectomy catheters [20]: The Angiojet system (Possis; Minneapolis, MN) uses the Venturi effect to perform thrombectomy. This is a double lumen catheter, of which the inner smaller catheter directs a high-velocity stream of saline. The high-pressure generated by the smaller lumen catheter creates a low pressure state in the larger catheter resulting in a vortex and promotion of fragmentation and aspiration of the thrombus.
Estimates of the efficacy of various interventional techniques are based on limited data, mostly case series. The overall clinical success rates, as measured by initial hemodynamic improvement, are reported as >70%. The reported mortality rates are wide, ranging from 0-30% across all techniques [24-28], although operator experience is clearly important in outcomes [29]. While many of these techniques may be employed without the use of systemic or local thrombolytics, it is notable that initial hemodynamic profiles may be superior when a thrombolytic agent is given concurrently [24]. Potential complications include pulmonary arterial perforation, pericardial tamponade, cardiac arrhythmias, pulmonary hemorrhage, pulmonary infarction and worsening hypotension from hemolysis [20,24].Question 5
What are the most recent American College of Chest Physicians (ACCP) guidelines on placement of IVC filters in pulmonary embolism?
- routine use of retrievable IVC filter in patients with PE
- use of IVC filter among patients with a contraindication to anticoagulation
- use of IVC filter among patients with recurrent PE despite adequate anticoagulation
- b and c
- all of the above
Answer to Question 5
Correct answer: D
The official recommendation from the 7th ACCP conference on Antithrombotic and Thrombolytic Therapy [30] is as follows: “In pulmonary embolism patients with a contraindication for, or a complication of anticoagulant therapy as well in those with recurrent thromboembolism despite adequate anticoagulation, we suggest placement of an IVC filter.”
Although this received only a Grade 2C recommendation (with low or very low evidence), there is general consensus within the pulmonary community that a patient at high risk for death due to recurrent pulmonary embolism may also benefit from placement of an IVC filter. This is based on a clinical trial of 400 patients with known deep vein thrombosis (with or without concomitant pulmonary embolism) randomized to IVC filter placement or anticoagulation alone. Concurrent placement of an IVC filter lowered the rate of new pulmonary embolism at day 12. There was no difference in PE rates at 2 years, although there was a higher incidence of DVT in the IVC filter group [31]. Although there was no difference in short-term mortality observed, patients with massive PE were not included in this study. Therefore, the use of a retrievable IVC filter [32] is a reasonable option among patients with severe hemodynamic compromise due to PE to prevent a recurrent catastrophic thromboembolism.
The patient required mechanically-assisted ventilation with a fraction of inspired oxygen (FiO2) of 0.6 and positive end-expiratory pressure (PEEP) of 10 cmH20 to maintain the arterial oxygen saturation >90%. Due to persistent hypotension after a trial of fluid resuscitation, norepinephrine was continued. A trial infusion of dobutamine was limited by prolonged runs of non-sustained ventricular tachycardia (NSVT). The patient’s urine output remained minimal. Interventional radiology placed an IVC filter but declined to perform a catheter thrombectomy due to the patient’s baseline depressed cardiac function.
Norepinephrine was discontinued by ICU day 6 and the patient’s oxygenation slowly improved, and mechanical ventilation was successfully discontinued on ICU day 8. Renal function improved without need for dialysis. Heparin was reintroduced before patient was discharged from the ICU without recurrence of hemoptysis. The patient recovered to her baseline status and was discharged on hospital day 39.
REFERENCES:
1. Kucher N and Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112: e28-e32.
2. Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med 2002; 136: 691–700.
3. Kasper W et al. Distinguishing between acute and subacute massive pulmonary embolism by conventional and Doppler echocardiography. Br. Heart J. 1993; 70: 352-6.
4. Molloy WD et al. Treatment of shock in a canine model of pulmonary embolism. Am Rev Respir Dis 1984; 130: 870-4.
5. Rosenberg JC et al. Isoproterenol and norepinephrine therapy for pulmonary embolism shock. J Thorac Cardiovasc Surg 1971; 62: 144-58.
6. Imamoto et al. Treatment of canine acute pulmonary embolic shock – effects of isoproterenol and norepinephrine on hemodynamics and ventricular wall motion. Jpn Circ J 1990; 54: 1246-57.
7. Manier G and Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis 1992; 145: 130-6.
8. Ducas J et al. Pulmonary vascular pressure-flow characteristics. Effects of dopamine before and after pulmonary embolism. Am Rev Respir Dis 1992; 145: 307-12.
9. Ghigone M et al. Volume expansion versus norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs. Anesthesiology 1984; 60: 132-5.
10. Jardin F et al. Dobutamine: a hemodynamic evaluation in pulmonary embolism shock. Crit Care Med 1985; 13: 1009-12.
11. Prewitt RM. Hemodynamic management in pulmonary embolism and acute hypoxemic respiratory failure. Crit Care Med 1990; 18: S61-9.
12. Wolfe MW et al. Hemodynamic effects of amrinone in a canine model of massive pulmonary embolism. Chest 1992; 102: 274-8.
13. Spence TH et al. Pulmonary embolism: improvement in hemodynamic function with amrinone therapy. South Med J 1989; 82: 1267-8.
14. Mercat A et al. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med 1999; 11: 339-45.
15. Piazza G and Goldhaber SZ. The acutely decompensated right ventricle. Chest 2005; 128: 1836-52.
16. Mebazaa A et al. Acute right ventricular failure – from pathophysiology to new treatments. Intensive Care Med 2004; 30: 185-96.
17. Layish DT and Tapson VF. Pharmacologic hemodynamic support in massive pulmonary embolism. Chest 1997; 111: 218-24.
18. Jamieson SW et al. Experience and results of 150 pulmonary thromboendarterectomy operations over a 29 month period. J Thorac Cardiovasc Surg 1993; 106: 116-27.
19. Gulba DC et al. Medical compared with surgical treatment for massive pulmonary embolism. Lancet 1994; 114: 576-7.
20. Uflacker, R. Interventional Therapy for Pulmonary Embolism. J Vasc Interv Radiol. 2001; 12:147-164
21. Gonzalez-Juanatey J et al. Treatment of massive pulmonary thromboembolism with low intrapulmonary dosages of urokinase. Chest 1992; 102: 341-6.
22. Verstaete M et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation 1998; 77: 353-60.
23. McCotter CJ et al. Intrapulmonary artery infusion of urokinase for treatment of massive pulmonary embolism: a review of 26 patients with and without contraindications to systemic thrombolytic therapy. Clin Cardiol 1999; 22: 661-4.
24. Kucher, N. Catheter embolectomy for acute pulmonary embolism. Chest 2007; 132: 657-663.
25. Greenfield LJ et al. Long-term experience with transvenous catheter pulmonary embolectomy. J Vasc Surg 1993; 18: 450-8.
26. Murphy JM et al. Percutaneous catheter and guidewire fragmentation with local administration of recombinant tissue plasminogen activator as a treatment for massive pulmonary embolism. Eur Radiol 1999; 9: 959-64.
27. Stock KW et al. Massive pulmonary embolism: treatment with thrombus fragmentation and local fibrinolysis with recombinant human-tissue plasminogen activator. Cardiovasc Intervent Radiol 1997; 20: 364-8.
28. Schmitz-Rode T et al. Fragmentation of massive pulmonary embolism using a pigtail rotation catheter. Chest 1998; 114: 1427-36.
29. Cela MC et al. Nonsurgical pulmonary embolectomy. In : Cope C, ed. Current Techniques in Interventional Radiology. Philadelphia: Current Medicine, 1994; 12: 2-8.
30. Buller HR et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126(3 Suppl):401S-428S.
31. Decousus H. et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998; 338(7):409-15.
32. Mismetti P et al. A prospective long-term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest 2007; 131: 223-9.
33. Leacche M et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018-23.