A Neonate with Progressive Respiratory Distress
Samantha W. Gee, MD (*)
Cameron Dezfulian, MD (†, *)
* Division of Critical Care Medicine, Department of Pediatrics, University of Florida/Shands Children’s Hospital, Gainesville, FL
† Division of Pulmonary, Sleep and Critical Care Medicine, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL
Case Presentation
A full term male infant born to a 16-year-old woman with regular prenatal care presented to his pediatrician at day of life four with the mother noting some subjective difficulty breathing. The pediatrician reassured the mother and gave them a subsequent follow up appointment. The mother and child then presented to the emergency department 2 days later for tachypnea and cyanosis, with diaphoresis and retractions exacerbated with feedings, while the child appeared more comfortable when upright.
The child was born full-term via a spontaneous vaginal delivery to a 16-year-old G1P1 woman with a pregnancy complicated only by gestational diabetes treated with diet control, and no prenatal infections (including negative Group B streptococcus screen).
His feeding amount had decreased slowly since birth and had stopped by the day of ER presentation. The child had rare sneezing and coughing but his mother had not noted fevers.
The child was not on any medications and had no prior allergies or surgery. The child resided with his mother, father, and grandparents who were all smokers. The grandmother recently had an upper respiratory infection. The family history was negative for congenital disease and otherwise non-contributory.
Physical Exam
The exam was notable for a well-developed tachypneic child in moderate respiratory distress at rest. The rectal temperature was 36.3ºC, the heart rate 133/min, the respiratory rate of 60-70/min, and the oxygen saturation of 65% on room air, improving to 92% with 1 LPM oxygen delivered with nasal prongs. The child’s weight was 3.54 kg (50th percentile), height 50 cm (50th percentile) and head circumference 34 cm (25th percentile). He had no dysmorphic features; the head, eye, ear nose and throat examinations were unremarkable. The child showed respiratory muscle retractions and displayed accentuated use of abdominal muscles to assist breathing. The chest examination was notable for decreased breath sounds on the left with scattered crackles and a clear right chest. Off oxygen, the child displayed central (lips) and peripheral (hands, feet) cyanosis, which improved with supplemental oxygen. The heart had a normal S1 and S2 with a regular rate and rhythm and no murmurs, rubs or gallops detected. The abdomen was non-distended and soft with normal active bowel sounds and no tenderness. Pulses were 2+ in all four extremities and the capillary refill was brisk. The child had normal male uncircumcised genitalia and the neurologic exam was normal with age-appropriate behavior.
Laboratory Findings
- WBC 14,700/mm3
- Hemoglobin 17.9 g/dL
- Normal electrolytes
- Normal coagulation panel
- C-reactive protein 2.6 mg/L
- Erythrocyte sedimentation rate 0 seconds
- Arterial blood gas (1/2 L nasal canula):
- pH of 7.34; PaCO2 of 50 mm Hg; PaO2 of 56 mm Hg
- Viral respiratory antigen swab of the nasopharynx:
- negative for influenza, parainfluenza and RSV
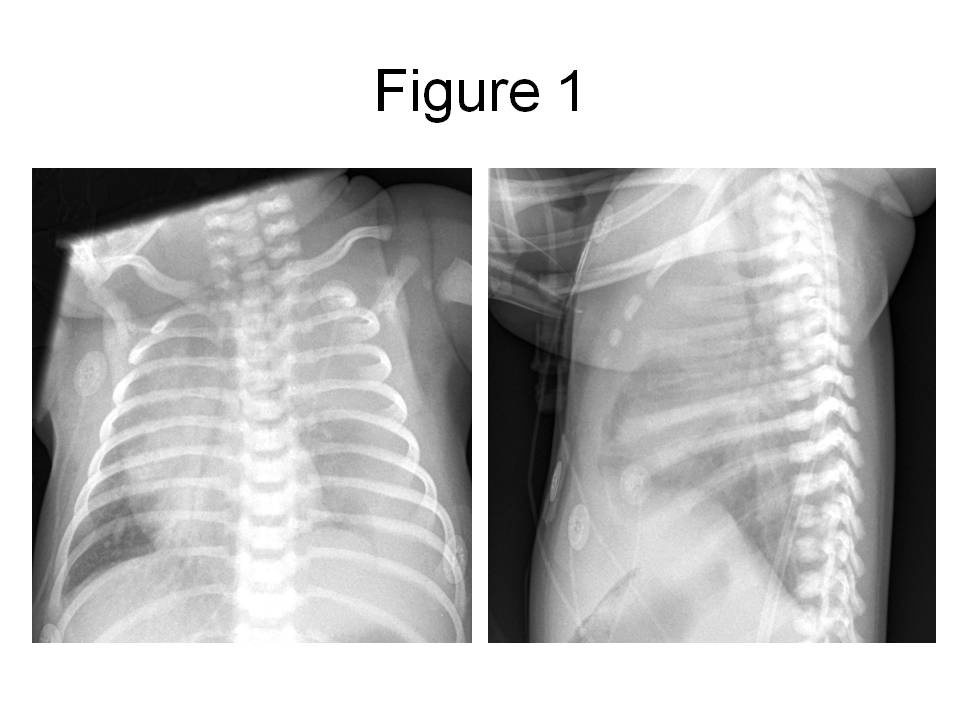
Figure 1. PA and lateral chest radiograph obtained in the emergency room, revealing a large left pleural effusion with displacement of the mediastinal structures into the right chest.
Hospital Course / Case Resolution
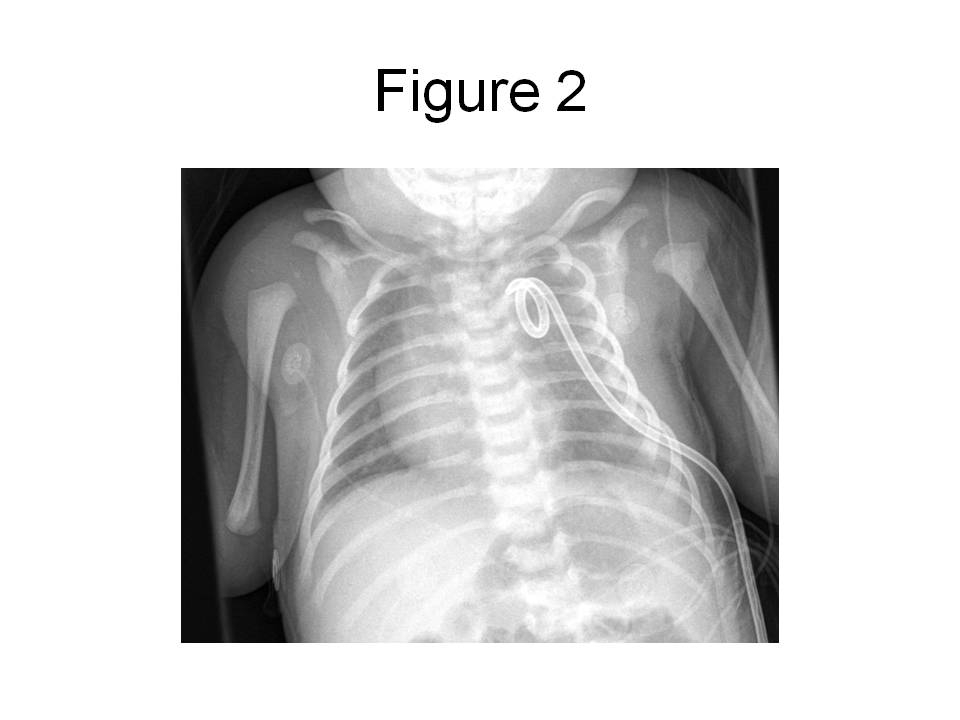
In the ER, a peripheral intravenous catheter was placed and empiric ampicillin and cefotaxime given. The child was transferred to the pediatric intensive care unit (PICU). In the PICU, the child was intubated for respiratory distress and a percutaneous tube thoracostomy was performed on the left side. The pleural fluid was grossly cloudy with a triglyceride level of 1075 mg/dL and 32,000 WBC/mm3 which were predominantly (90%) lymphocytes. The protein content of the fluid was 2.9 g/dL and the LDH was 307 U/L. Gram’s stain of the fluid revealed 2+ WBC and the cultures remained negative for 72 hours, at which point antibiotics were discontinued. The child’s chest radiograph after tube placement revealed resolution of the effusion with no evidence of intrapulmonary masses (see figure 2) and the child was subsequently extubated.
Figure 2. Portable (AP) chest radiograph obtained in the PICU after chest tube placement and extubation reveals complete resolution of the pleural effusion.
An echocardiogram revealed no abnormalities of the heart with normal cardiac function. Genetic screening did not reveal chromosomal abnormalities. The child was fed with a low fat formula and within approximately 10 days the drainage from the chest tube became negligible and it was removed. The child did not reaccumulate an effusion in the subsequent days and was discharged home on this formula.
Question 1
Of the following, which is the BEST answer for how a chylothorax is distinguished from other causes of pleural effusion?
- Ultrasound echotextures
- Chest CT with classic absorption in discrete Hounsfield unit distribution
- Milky appearance of pleural fluid
- Serum triglyceride level
- Pleural fluid triglyceride level
Answer to Question 1
Correct answer: E
Chylothorax is a fluid mixture composed of fats (triglycerides, phospholipids, and cholesterol), proteins (albumin, fibrinogen, prothrombin, and immunoglobulins), lymphocytes, fat-soluble vitamins, and electrolytes1,2. Chyle is the result of the intestinal absorption of fats, primarily in the form of triglycerides, and is carried by chylomicrons through the lymphatic system. Without minimal intake of fat enterally, the classic milky appearance of chyle is less obvious. Thoracentesis and aspiration of fluid classically demonstrates a turbid, milky-appearing fluid if a patient is feeding. Neonates who are not yet on enteral feeding may not have this characteristic milky appearance and the fluid may in fact be clear, as explained previously. Evaluation of chylothorax reveals the following prominent characteristics, provided that the sample has ≥ 1000 cells/µL3,4:
- Triglyceride level > 110 mg/dL
- Lymphocytes 400-6800/mm3 (or 80-100% lymphocyte predominance)
- pH 7.4-7.8
- Cholesterol 65-220 mg/dL
- Total protein 2.2-5.9 g/dL
- Albumin 1.2-4.1 g/dL
- Erythrocytes 50-600/mm3
Aside from neonates with hydrops fetalis, pleural effusions in this age group are rare. The differential diagnoses and estimated incidences are summarized below4
|
Cardiac: congenital heart disease leading to congestive heart failure |
19-26% |
|
Pulmonary: chylothorax, lymphangiectasia, cystic adenomatoid malformation or sequestration |
8-10% |
|
Genetic: trisomy 21, 18, 13, 15, 16, Turner syndrome |
35% |
|
Fetal anemia: maternal-fetal hemorrhage, α-thalassemia |
10-27% |
|
Infectious: cytomegalovirus, coxsackie virus, toxoplasmosis, syphilis, parvovirus B19, rubella, respiratory syncytial virus, herpes simplex, varicella |
1-8% |
As in this case report, most neonatal effusions present with acute respiratory distress. Neonates may present at birth with a clinical picture of respiratory failure necessitating evacuation of the fluid once the patient is stabilized with mechanical ventilation2. In cases of chylothorax, a thoracostomy tube is often needed, as drained fluid can recur within less than a day and again compromise full lung expansion. If chylothorax occurs in utero prior to 33 weeks gestation there is an associated risk of increased infant mortality secondary to the development of hypoplastic lungs1. Congenital chylothorax, associated with certain birth abnormalities including trisomy 21, hydrops fetalis, sex chromosome mosaicism, Turner syndrome, and Noonan syndrome has an even higher mortality, up to 60% of such pregnancies1,3,4,5.
Question 2
Of the following, which is NOT a known cause of neonatal chylothorax?
- Heart failure
- Liver failure
- Lymphangiomatosis
- Central venous access
- Shaken baby syndrome
Answer to Question 2
Correct answer: B
Chylothorax in neonates is linked to either an original anatomic abnormality or to resultant damage to the thoracic duct by various processes, both iatrogenic and consequential to disease. Examples of congenital malformations include lymphangiectasias and lymphangiomatosis, as well as the lymphatic malformations that are associated with trisomy 21 and Noonan syndrome3. Given that the lymphatic system and its many collateral vessels span the length from the cisterna chyli in the abdomen to the thoracic duct near the subclavian, the occurrence of mechanical trauma to the ducts is not uncommon from any surgery. In particular, the apparent rise in incidence of chylothoraces in neonates is partly due to the fact that surgical improvements have been made in the field of congenital heart disease.
In addition chylothorax is a known complication of central venous line placement, where the impedance of venous flow and venous return results in increased central venous pressures. This increase in venous pressure translates into higher pressures in the lymphatic system and subsequent development of chylothorax6. A similar phenomenon can occur when either the left of right subclavian vein is thrombosed, presumably from the local increase in pressure as well3.
Finally, isolated chylothorax in an otherwise healthy infant may be secondary to non-accidental trauma7,8. The mechanism of blunt trauma to the chest or of forceful acceleration-deceleration types of motion may be significant enough to rupture the thoracic duct. Further investigation with a full skeletal survey, and any additional studies that are clinically indicated for evaluation of non-accidental trauma, is warranted.
Question 3
Which of the following statements regarding the pathophysiology of chylothorax is INCORRECT?
- Chylothoraces in premature babies can occur when there is compression of the main thoracic duct.
- Chylothoraces can occur when there is an overabundance of lymphatic vessels in the body.
- Chylothoraces can occur in the absence of trauma to the lymphatic duct.
- Chylothoraces that occur unilaterally are due to inadequate lipid intake.
- Chylothoraces may occur after complex congenital heart surgeries
Answer to Question 3
Correct answer: D
The etiology of chylothorax can be simplified into one of two major mechanisms: disrupted development of the native lymphatic system or a functional obstruction to chylous flow. Examples of disrupted or abnormal development of the lymphatic system include lymphangiomatosis and lymphangiectasia, where functional obstructions may arise from complications of thoracic surgical procedures, pleural and mediastinal malignancies or from a pathological increase in systemic venous pressure related to congenital heart disease.
Regarding the native lymphatic system, it is unclear as to how or when certain disruptions or failure of interruptions occur in the development of the lymphatic system. Lymphangiomatosis, also previously known as cystic hygromas or lymphangiomas, is characterized by the sequestration of lymphatic tissue and the resultant inability of the body to adequately drain the added lymph produced by this tissue. In lymphangiectasia, there is an overabundance of subpleural and interlobular lymphatic vessels with an associated inability of the body to adequately drain lymph effectively1.
Mechanical injury to the lymphatics may be unavoidable in complex surgeries. Any physical disruption along any portion of the thoracic duct or its smaller tributaries may contribute to the formation of a chylothorax. As the thoracic duct courses the thoracic cavity in the posterior mediastinum, it occupies a unique position between the aorta and the azygos vein to the level of T5 crossing over to the left chest cavity behind the aortic arch and finally terminating as the thoracic duct. Chylothorax is a relatively common complication of corrective surgery for tetralogy of Fallot, total anomalous pulmonary venous return and transposition of the great vessels.
Finally, malignancies and unbalanced venous pressures may also contribute to the development of chylothoraces in the newborn. Due to mass effects or the direct invasion of disease into lymphatic vessels, thoracic malignancies such as neuroblastoma and lymphomas are the original etiology of some chylothoraces1. As noted in the prior question any increase in systemic venous pressure will transmit into higher lymphatic pressures and leakage of fluid; therefore, both unrepaired and repaired congenital heart disease patients are at risk.
Question 4
What is the MOST appropriate next step in the management of a non-traumatic, uncomplicated chylothorax?
- Total parental nutrition for 6 weeks
- A trial of diuretics
- A trial of formula containing medium-chain triglycerides
- Infusion of albumin for three days
- Switching to smaller volume, more frequent feeds
Answer to Question 4
Correct answer: C
After drainage of the chylothorax and resolution of respiratory symptoms, a 2-3 week trial of a medium-chain triglyceride (MCT) formula with close observation for reaccumulation of fluid, is a common approach9. However, there is no accepted standard of care and some have advocated the complete cessation of enteral feeds for 2-4 weeks with the institution of total parenteral nutrition during that time period. Most centers favor the use of MCT formulas when feeds are restarted since MCTs are directly absorbed into the portal venous system rather than utilizing the lymphatic drainage and thus decrease overall lymph production. If chylothorax is the result of cardiac failure and high systemic venous pressures, therapy to treat this underlying cause (diuretics, salt restriction) is most appropriate.
Management of refractory cases of chylothorax, recurring despite conservative treatment for 2-5 weeks, can be more challenging3,4,9. Surgical intervention is indicated if the thoracic duct has a leak, with ligation being the primary surgical repair performed4. However, duct ligation is not always definitive due to the variations in ductal anatomy that allow for “collaterals” of lymph ducts to effectively avoid the area ligated3. In cases where the thoracic duct is deemed to be intact, a pleurodesis or pleuroperitoneal shunt may be indicated for definitive treatment1,4.
Interestingly, there are also an increasing number of cases in the literature that promote the successful use of octreotide for 1-2 weeks, as a continuous infusion5,10,12. The proposed mechanism of action is vasoconstriction exerted on the splanchnic circulation with a secondary decrease in lymph production in the gastrointestinal system. Octreotide therapy may be an appealing alternative to surgery and can be attempted for chylothoraces due to various underlying etiologies.
Question 5
Which of the following is a common complication of chronic chylothorax?
- Kwashiorkor
- T cell deficiency
- Hypernatremia
- Pulmonary hypertension
- Hypoglycemia
Answer to Question 5
Correct answer: B
Notwithstanding the inherent complications that accompany persistent fluid loss, significant and persistent chylothoraces place patients in a unique immunological risk category. This is due to the large leakage of T lymphocytes and immunoglobulins that occurs with chylothorax1,11. Laboratory evaluation of immunoglobulin levels is warranted, and periodic supplementation with intravenous immunoglobulin may be required.
References
- Dubin PJ, King IN, and Gallagher PG. Congenital chylothorax. Curr Opin Pediatr 2000 Oct, 12(5):505–509.
- Straaten, HL, Gerards LJ, Krediet TG. Chylothorax in the neonatal period. Eur J Pediatr 1993 Jan;152(1):2-5.
- Buttiker V, Fanconi S, Burger R. Chylothorax in children: Guideline for diagnosis and management. Chest 1999; 116:682-687.
- Rocha G. Pleural effusions in the neonate. Curr Opin Pulm Med 2007; 13:305-311.
- Siu SL, Lam DS. Spontaneous neonatal chylothorax treated with octreotide. JPediatr Child Health 2006; 42:65-67.
- Kurecki E, Kaye R, Koehler M. Chylothorax and chylopericardium: a complication of a central venous catheter. J Pediatr 1998; 132:1064.
- Anderst JD. Chylothorax and child abuse. Pediatr Crit Care Med 2007; 8:394 –396.
- Geismar SL, Tilelli JA, Campbell JB, Chiaro JJ. Chylothorax as a manifestation of child abuse. Pediatr Emerg Care 1997; 13:386.
- Orenstein, DM. Diseases of the pleura. In: Behrman RE, Kleigman RM, Jenson HB, editors. Nelson textbook of pediatrics, 16th ed. Philadelphia: WB Saunders; 2000. pp. 1333-1334.
- Rasiah SV, Oei J, Lui K. Octreotide in the treatment of congenital chylothorax. J Pediatr Child Health 2004; 40:585-588.
- Goto M, Kawamata K, Kitano M, Watanabe K, Chiba Y. Treatment of chylothorax in a premature infant using somatostatin. J Perinatol 2003 Oct;23(7):563-564.