Kamalbir Dhaliwal, MD
Resident, Department of Medicine
Southern Illinois University School of Medicine
AND
Akshay Sood, MD, MPH
Assistant Professor of Medicine, Division of Pulmonary and Critical Care Medicine
Southern Illinois University School of Medicine
A 35-year-old white male with no significant past medical history was transferred from a small rural town in Illinois to the Regional Burn Unit at the University Hospital. The patient was a farmer's son and a 20 pack-year smoker. He was involved in the illicit manufacture of methamphetamine in the basement of his home. Subsequent explosion resulted in 16% total body surface area burn involving his face, neck and hands, along with difficulty with breathing and vision.
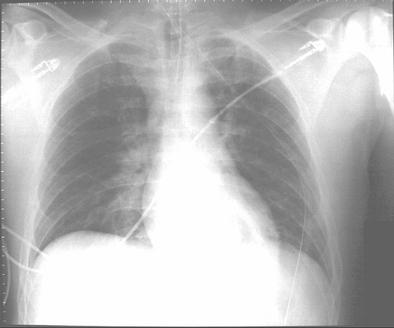
Before transfer to the Burn Unit, the paramedics intubated the patient in the field and irrigated his eyes with water. A nasogastric tube and an indwelling urinary catheter were also placed. His examination at the Burn Unit showed conjunctival erythema, lacrimation, thick nasal discharge, wheezing, pharyngeal erythema and second-degree skin burns (see Figure 1). His initial laboratory reports were within normal limits. His arterial blood gas drawn on Assist Control mode of ventilation with a tidal volume of 8 cc/kg body weight, set respiratory rate of 12/min with patient rate at 14/min, PEEP of 5 and FiO2 of 40% showed a pH of 7.48, a PaCO2 of 32 mmHg and a PaO2 of 70 mmHg. His chest X-ray portable anteroposterior view is shown in Figure 2.
Click on images to enlarge.
Figure 1. Photograph of skin burns.
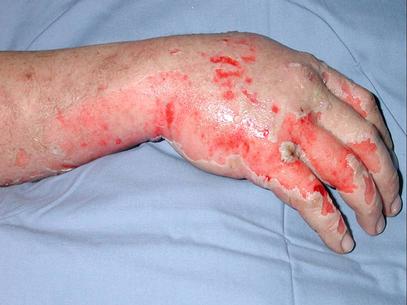
Figure 2. Chest X-ray portable anteroposterior view.
REFERENCES:
- Wibbenmeyer LA, Morgan LJ, Robinson BK. Our chemical burn experience: exposing the dangers of anhydrous ammonia. J Burn Care Rehabil 1999; 20(3): 226-231.
- Millea TP, Kucan JO, Smoot EC, 3rd. Anhydrous ammonia injuries. J Burn Care Rehabil 1989; 10(5): 448-453.
- Montague TJ, Macneil AR. Mass ammonia inhalation. Chest 1980; 77(4): 496-498.
- Summer W, Haponik E. Inhalation of irritant gases. Clin Chest Med 1981; 2(2): 273-287.
- Close LG, Catlin FI, Cohn AM. Acute and chronic effects of ammonia burns on the respiratory tract. Arch Otolaryngol 1980; 106(3): 151-158.
- O'Kane GJ. Inhalation of ammonia vapour. A report on the management of eight patients during the acute stages. Anaesthesia 1983; 38(12): 1208-1213.
- Arwood R, Hammond J, Ward CG. Ammonia inhalation. J Trauma 1985; 25(5): 444-447.
- Birken GA, Fabri PJ, Carey LC. Acute ammonia intoxication complicating multiple trauma. J Trauma 1981; 21(9): 820-822.
Question 1
Which ONE of the following is a possible cause of chemical inhalational lung injury during production of illicit methamphetamine?
(a) Ammonia
(b) Chlorine
(c) Ozone
(d) Phosgene
(e) Mustard Gas
Answer
The correct answer is A.
Clandestine methamphetamine laboratories are mushrooming across the United States, particularly in the rural farming areas. These laboratories are typically small, utilizing household appliances and readily available chemicals. The key ingredient that limits illicit methamphetamine production is anhydrous ammonia. This compound is often stolen from tanks left in farmers' fields and from wholesale ammonia dealers and drained into smaller tanks that may explode when heated. Subsequent exposure to anhydrous ammonia can cause serious injury to the respiratory tract, and even death. Among chemical burn patients admitted to the hospital, about a third result from anhydrous ammonia exposure (1, 2).
Other common exposure settings involve release of "fumes" from a variety of household cleaning agents at home. Ammonia is also present in swine confinement buildings and is used as a soil fertilizer on farms. Accidents involving the commercial use of refrigeration equipment and in the plastics, textiles, leather, explosives and fertilizer industries are other exposure settings.
Question 2
Which ONE of the following BEST describes the pathophysiology of the tissue injury caused by this chemical?
(a) Saponification of fats and liquefactive necrosis
(b) Binding to enzyme cytochrome oxidase, thereby halting aerobic metabolism
(c) Direct irritant effect on mucosa
(d) Reduction in oxygen-carrying capacity of hemoglobin
(e) Thermal injury
Answer
The correct answer is A.
Ammonia reacts with water present in moist mucosal surfaces to form ammonium hydroxide, a potent alkali. This causes saponification of the lipids found in cell membranes. This leads to liquefactive necrosis of the mucosal surface, which in turn, exposes more ammonia to the watery surface and results in further necrosis. This reaction NH3+H2O NH4OH is exothermic and can also cause thermal injury. The tissue penetration of ammonia is thus deeper compared with equipotent acid. Anatomic areas most commonly involved are the skin, respiratory system, and ocular structures (2).
Question 3
Which ONE of the following is not seen during the bronchoscopic examination of this patient's airways?
(a) Edematous trachea and mainstem bronchi
(b) Erythematous trachea and mainstem bronchi
(c) Sloughing of mucosa to form thick coagulum
(d) Vesicles involving the mucosa
(e) Excessive tracheobronchial secretions
Answer
The correct answer is D.
The most frequent injury to the respiratory tract is desquamation of the mucosal epithelial layer. The destruction of respiratory mucosa involves edema, erythema, hemorrhagic areas, and sloughing of the mucosa to form thick coagulum. In addition, abundant secretions are also described. Chest roentgenographic findings at presentation may correlate poorly with respiratory tract involvement (3).
Video 1: Bronchoscopic picture following ammonia inhalation
(320KB)
(Please right-click and save file to view with QuickTime Player.)
Question 4
Which ONE of the following statements is false?
(a) Patient with facial or oral lesions are at high risk for developing laryngeal edema
(b) Intubation with a large size endotracheal tube is warranted
(c) Lower airway is affected more than upper airway
(d) Gastrointestinal tract may be affected
(e) Pulmonary edema may be delayed
Answer
The correct answer is C.
The severity of injury depends upon the concentration and duration of the exposure. Because of its' high water solubility, it affects upper airways more than lower airways. Ammonia is markedly irritating to the eyes, skin and mucous membranes of upper respiratory tracts and may cause severe edema of the larynx, resulting in fatal upper airway obstruction. Chemical bronchitis, bronchospasm, and noncardiogenic pulmonary edema are also described with more intense exposures. Furthermore, while the oppressive odor of the gas would tend to drive an individual away from the scene, blindness resulting from the exposure may trap the patient in the area and assure more severe respiratory injury (4).
Question 5
Which ONE of the following may NOT be useful in the management of this patient?
(a) Removal of contaminated clothing
(b) Treating skin burns with copious amounts of water
(c) Systemic corticosteroids
(d) Bronchodilators
(e) Fluid restriction
Answer
The correct answer is C.
Initial treatment is primarily supportive and includes removal of the patient from exposure, removal of contaminated clothing, administration of supplemental oxygen, washing of exposed areas with copious amounts of water and irrigation of eyes with water. Individuals with orofacial lesions may be at higher risk of developing acute laryngeal obstruction; however, this early complication can develop even in patients without visible injury. Patients with postexposure abnormalities clinically or on chest radiograph, pulmonary function test or increased arterial alveolar gradient on blood gas analysis should be observed in the hospital for at least 24 hr.
Some authors advise early tracheal access because of the risk of obstruction (5, 6). Use of large-sized endotracheal tube is recommended to prevent plugging by sloughed mucosa. The use of corticosteroids is controversial and may increase infectious complications (6). They may play a role in severe bronchospasm (7). Lesser degree of bronchospasm may be treated with inhaled bronchodilators (7). The use of antibiotics is also controversial: the high risk of pulmonary superinfection in these patients (8) needs to be balanced against the risk of selection of multiresistant pathogens (7). Clinicians need to be aware of fluid over-resuscitation because of the risk of noncardiogenic pulmonary edema.
Question 6
Which ONE of the following is LEAST likely to be a late sequela of this exposure?
(a) Reactive airways dysfunction syndrome (RADS)
(b) Bronchiolitis obliterans
(c) Pulmonary fibrosis
(d) Bronchiectasis
(e) Delayed encephalopathy
Answer
The correct answer is E.
Complete recovery is usual but persistent effects following an acute inhalation include chronic cough and hoarseness, reactive airways dysfunction syndrome (RADS), bronchiectasis, airway stenosis, and bronchiolitis obliterans/bronchiolitis obliterans organizing pneumonia. Delayed encephalopathy seen with carbon monoxide and hydrogen sulfide poisoning is not seen with ammonia inhalation.



