Tabuchi A, Styp-Rekowska B, Slutsky AS, Wagner PD, Pries AR, Kuebler WM. American Journal of Respiratory and Critical Care Medicine 2013; 188(4):474-481
Background: Concepts of gas exchange dynamics at the capillary level have been based on the physical and chemical properties of gas diffusion as well as anatomic information. This is the first in vivo mammalian study to investigate this fundamental issue by directly probing gas exchange at the alveolar-capillary level. If successful, the results could alter how we conceptualize oxygen exchange and may be useful in further understanding the pathogenesis of pulmonary hypertension.
Methods: Three imaging techniques were combined to directly measure oxygen uptake dynamics in intact mice. Intravital microscopy was performed through a surgically excised thoracic window in anesthetized mice. This is a technique in which fluorescence is excited in the tissue of interest and light emitted from it is collected without interference from surrounding tissues. Multispectral oximetry was conducted, using changes in the absorption spectrum of hemoglobin based on the oxygen saturation. Lastly, blood flow velocity was measured by double-strobe spatial correlation. These techniques were performed on intact anesthetized male BALB/c mice, as well as mice exposed to hypoxic conditions (10% O2) for 6 weeks.
Results: Oxygen saturation maps were constructed under normoxic, hyperoxic, and hypoxic conditions; there was a near-perfect correlation between spectrometry-derived in vivo saturations and oxygen values from systemic blood gas analysis. During normoxic ventilation, significant pre-capillary oxygenation occurred in small pulmonary arterioles prior to entering the capillaries; blood entered the larger pulmonary arterioles at a saturation of ~70% and oxygenation increased to ~85% within the terminal branches of the arterioles. In fact, oxygen saturation levels in pulmonary arterioles increased exponentially with decreasing vessel diameter, with marked oxygenation occurring in arterioles <30µm in diameter. Individual RBC pathways from pulmonary arterioles to venules were identified, and rapid oxygen uptake occurred in the pre-capillary arterioles, accounting for 50% of the total increase in oxygen during RBC transit. Lastly, to investigate the effect of pulmonary arteriole remodeling on oxygen dynamics, mice subjected to 6 weeks of hypoxia were studied. As expected, this led to significant arteriolar medial wall thickening, which resulted in lower oxygen saturation values in pre-capillary arterioles. This phenomenon was not due to lower mixed venous oxygen saturation or erythrocytosis, but instead was caused by impaired oxygen uptake in the thickened small arterioles.
Conclusions: By directly probing the subpleural microcirculation of intact anesthetized mice, this study demonstrates that oxygenation begins in pre-capillary arterioles and that 50% of oxygen uptake occurs before the RBCs ever enter the capillary network. This pre-capillary oxygenation was attenuated in mice with remodeled pulmonary arterioles. If the results from this murine model can be accurately extrapolated to the human condition, this may further our understanding of impaired oxygenation in patients with pulmonary hypertension.
Commentary: In this article the first measurements of oxygen saturation in pulmonary arterioles are reported. Understanding the details of the rate and location of oxygen uptake in the pulmonary microcirculation provides important insights into the processes of gas exchange. The data presented here apply specifically to the boundaries of gas exchange, i.e. oxygen uptake begins in the arteries upstream of the capillary bed. The boundaries of gas exchange were previously deduced by a number of clever but indirect studies. The current breakthrough opens the possibility for important investigations of physiologic and pathophysiologic changes in the pulmonary microcirculation. Here, for example, they have shown that oxygen uptake in arterioles was impeded in animals exposed to prolonged hypoxia with the attendant thickening of arteriolar walls. With many assumptions, this finding could have been predicted from Fick’s first law of diffusion, but now we have direct measurements. Future studies could investigate diseases in which the capillary bed is reduced, an alteration that could shift the gas exchange load even farther upstream in precapillary vessels.
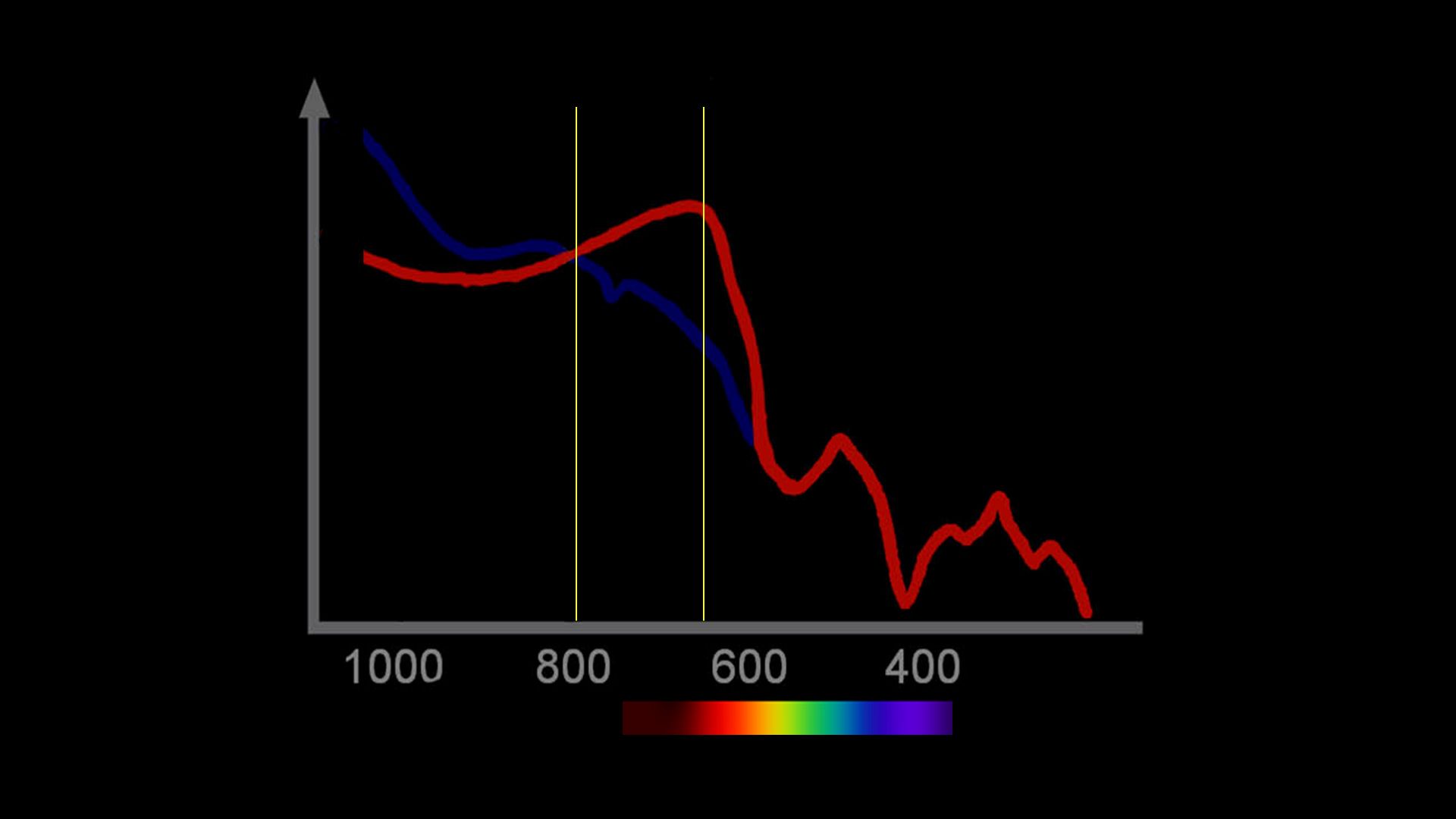
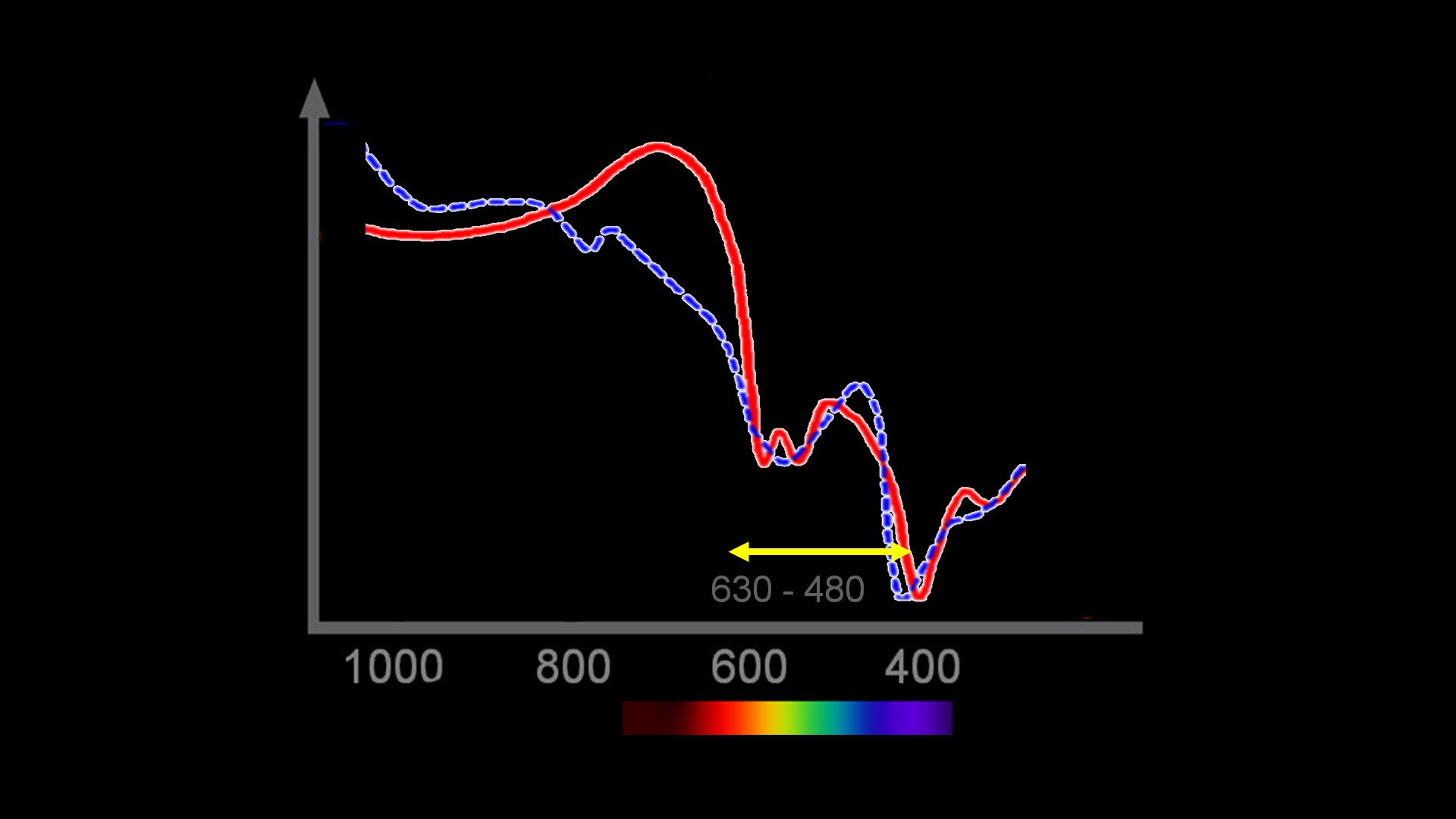
Historically, this is not the only attempt to make these difficult measurements. To understand the problem, a few basics are needed. When light is reflected from blood, the intensity is dependent on the concentration of the blood, thickness of the sample, and the color. The last of these variables, the color, is the important one for it directly reflects the oxygen saturation (Figure 1). The problem is to somehow account for the concentration and thickness and measure only the color. Traditionally this is done by measuring the light at a specific wavelength that is insensitive to the color differences of hemoglobin oxygen saturation. This wavelength, therefore, measures concentration and thickness, but not color. A second wavelength is used that measures concentration and thickness, and is chosen to have a maximal difference between the color of oxy- and reduced hemoglobin. By combining these measurements, the intensity of the color alone can be determined and related to oxygen saturation (Figure 2).
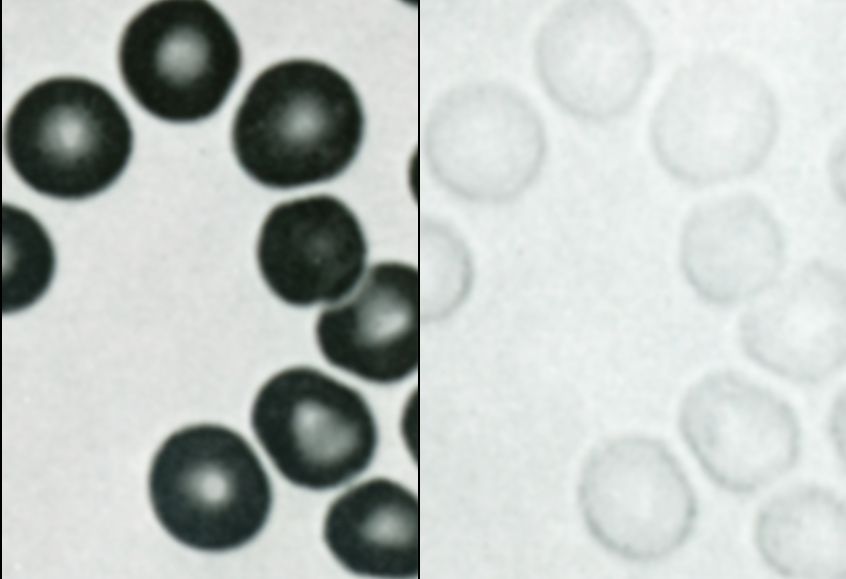
Here is the problem with microvessels. The microscopic blood samples are so thin that they permit almost all of the light to pass through blood without reflecting much signal. This difficulty has stymied all progress in the past. In the late 1960’s when my laboratory tackled this problem, we were limited by available technology (Figure 3). After a decade of trying each of the newest updated detectors, none of which worked, our group was forced to give up. Since then no one else has succeeded in making these measurements in the pulmonary microcirculation. Although technology advanced rapidly in the following years, it is only now that detectors, computers, and software have become available so that these measurements have finally become feasible. Nevertheless the problem has been far from trivial. The investigators have been able to incrementally scan microvessels over much of the visible spectrum collecting data at 75 different wavelengths. Reflected wavelengths from the faint signals from oxy- and reduced hemoglobin were recorded as the spectral curves crisscrossed. They developed impressive software that could tease these signals apart, and, in nothing less than a technical tour de force, have been able to measure oxygen saturation in precapillary arterioles for the first time.
Article summary by: Matthew Lammi, MD; Assistant Professor of Medicine, Louisiana State University Health Sciences Center
Expert commentary by: Wiltz W. Wagner, Jr., PhD; Professor of Pharmacology, University of South Alabama College of Medicine
Figure Legends
Figure 1: Spectral range with an isosblestic point at 805 and a color signal at 660. This is the pair used in the finger pulse oximeter.
Figure 2: Spectral range used in the present paper. Note that there are many isosblestic points and color signal points. By moving over the spectrum, the investigators could tease out the saturation from the signal ratios.
Figure 3: Photomicrographs published in 1969 by Dr. Wiltz Wagner. These show the same red blood cells at 404 (violet; Left) and 578 (yellow; Right).