William S. Beckett, M.D., M.P.H.
Professor of Environmental Medicine and Medicine
University of Rochester School of Medicine and Dentistry
A 48 year old white male supervisor, employed at a plant which contracts to apply metal coatings to pre-manufactured metal parts, was admitted to a tertiary care hospital with a three-year history of cough and progressive exertional dyspnea. He noted a several-year history of rash involving the peroneal aspects of both lower extremities.
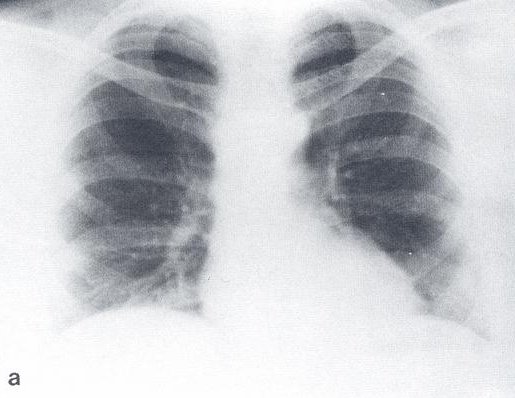
His past medical history was significant for an episode of urolithiasis with spontaneous passage of the stone, and mild hypertension treated with a beta-blocker. He had no childhood history of respiratory disease or chest trauma and was a lifelong non-smoker. Chest X-Ray taken for tuberculosis screening at age 29 years (Figure 1) was unremarkable. There was no family history of interstitial and other lung disease.
Click on images to enlarge.
Figure 1: Chest X-ray 19 years prior to presentation
He served in the United States Army for two years as a radio operator. He then went to work at age 25 for his current employer, where he worked for 10 years as a metallurgic technician and a line foreman in the metal parts coating operation where coatings of tungsten carbide, cobalt, copper, nickel, and other metals were applied to metal parts using the detonation gun and plasma coating processes. He then worked for five years as a grinder using a diamond wheel to grind the metal-coated parts. He gave no history of exposure to asbestos dust. In the ten years prior to presentation, he was employed primarily in management, with little dust exposure.
Physical examination was remarkable for diffuse crackles heard anteriorly and posteriorly on a chest exam, with crackles more pronounced on the right.
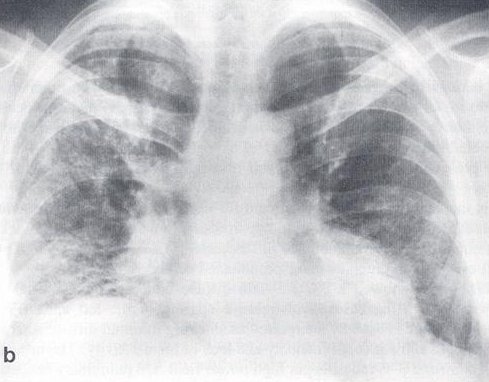
Figure 2: Chest X-ray at presentation
Chest X-ray done at presentation to the hospital showed a bilateral and diffuse increase in interstitial markings (Figure 2). The urine contained trace protein and 3-5 red cells per high power field. On pulmonary function testing, the forced vital capacity (FVC) was 2.07 liters (46% predicted); forced expiratory volume in one second (FEV1) of 1.66 liters, the ratio of FEV1/FVC was 80%; total lung capacity, functional residual capacity, and residual volume were approximately 45% of predicted, and diffusing capacity was 35% predicted. On room air, arterial blood gases showed pH 7.45, pCO2 38 mm Hg, and pO2 90 mm Hg. The hematocrit was 56%, white cell count 8,200 cells/mm3 with 63% polymorphonuclear cells, 31% lymphocytes, 5% monocytes and 1% eosinophil. Erythrocyte sedimentation rate was 7 mm/hr (0-15), and Angiotensin converting enzyme level was 16 units/ml (12-35). Anti-nuclear antibody was negative. Liver function tests were within normal limits.
Selected Readings (Please click on the hyperlink):
- NIOSH Pocket Guide to Chemical Hazards on cemented tungsten carbide
- NIOSH Criteria Document 77-127 (September 1977) - Criteria for a recommended standard: Occupational Exposure to Tungsten and Cemented Tungsten Carbide [9]
- OSHA Guideline for Cobalt metal, dust, and fume
REFERENCES:
- Sprince, N.L., et al., Cobalt exposure and lung disease in tungsten carbide production. A cross-sectional study of current workers. Am Rev Respir Dis, 1988. 138(5): p. 1220-6.
- Lison, D., et al., Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur Respir J, 1996. 9(5): p. 1024-8.
- Villar, T.G., Vineyard sprayer's lung. Clinical aspects. Am Rev Respir Dis, 1974. 110(5): p. 545-55.
- Nemery, B., E. Verbeken, and M. Demedts, Giant cell interstitial pneumonia (Hard metal lung disease, cobalt lung). Sem Resp Crit Care Med, 2001. 22(4): p. 435-447.
- Ohori, N.P., et al., Giant-cell interstitial pneumonia and hard-metal pneumoconiosis. A clinicopathologic study of four cases and review of the literature. Am J Surg Pathol, 1989. 13(7): p. 581-7.
- Michetti, G., et al., Bronchoalveolar lavage and its role in diagnosing cobalt lung disease. Sci Total Environ, 1994. 150(1-3): p. 173-8.
- Davison, A.G., et al., Interstitial lung disease and asthma in hard-metal workers: bronchoalveolar lavage, ultra structural, and analytical findings and results of bronchial provocation tests. Thorax, 1983. 38(2): p. 119-28.
- Demedts, M., et al., Cobalt lung in diamond polishers. Am Rev Respir Dis, 1984. 130(1): p. 130-5.
- Criteria for a recommended standard: Occupational exposure to tungsten and cemented tungsten carbide. 1977, DHHS.
- Harding, S.M., Woman with dry cough and dyspnea on exertion has clubbing, conjunctival injection, and diffuse crackles. Chest, 2003. 123(3): p. 935-6.
- Cugell, D.W., et al., The respiratory effects of cobalt. Arch Intern Med, 1990. 150(1): p. 177-83.
Acknowledgement:
Permission to reproduce the case has been obtained from "American Journal of Industrial Medicine copyright 1992."
Question 1
Which of the following occupational metal exposures is LEAST likely to cause interstitial lung disease?
a) Tungsten
b) Cobalt
c) Copper
d) Nickel
e) Steel
Answer
The correct answer is E.
Interstitial lung disease that has been described to be associated with tungsten and cobalt is also called hard metal lung disease. Tungsten carbide, the "hard-metal" that gives this disease its name, is made by mixing minute particles of tungsten, carbon, and up to 25% cobalt, and then 'sintering' the mixture at high temperature to produce an extremely hard and heat-stable alloy. The component of hard metal causing this disease is thought to be cobalt. Even though tungsten alone is not believed to play a major role in the pathogenesis of this disease, the combination of cobalt and tungsten may be worse than cobalt alone [1] [2].
Repeated or chronic exposure to nickel carbonyl may induce pulmonary fibrosis. A rare interstitial lung disease resembling silicosis, also called vineyard sprayer's lung, is associated with copper exposure and is seen only among agricultural workers in Portugal. This disease appears to be related to inhalation of Bordeaux mixture, which is a 1 to 2.5% solution of copper sulfate neutralized by hydrated lime [3]. Bordeaux mixture is applied manually to combat mildew on grape vines. Even though 'siderosis' is described following chronic exposure to iron dust, iron fume and iron oxide dust, steel is not thought to be a major cause of lung disease.
Question 2
The patient subsequently underwent a transbronchial biopsy of the lung which showed a distortion of alveolar architecture by fibrotic thickening of the septae, moderate interstitial inflammation, numerous macrophages within alveoli and hyperplastic type II pneumocytes diffusely. In addition, several 'bizarre' appearing cells were seen, similar to those seen in Figure 3, which was obtained from an open lung biopsy from a similar patient.
FIGURE 3: Several 'bizarre' appearing cells seen in this open lung biopsy specimen from a similar patient.
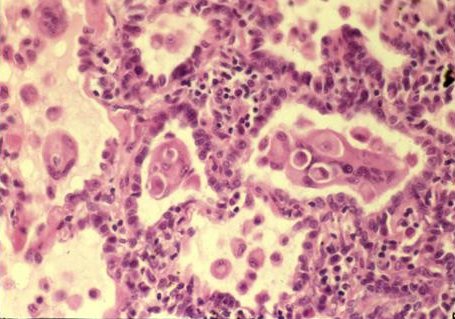
Courtesy of E. Verbeken and B. Nemery, University of Leuven, Belgium
Figure 3 demonstrates which classical finding associated with this disease?
a) Reed Sternberg cells
b) Large cell cancer
c) Multinucleated Giant cells
d) Owl's eye nucleus
e) Erythrophagocytosis
Answer
The correct answer is C.
Figure 3 demonstrates the characteristic pathologic finding of multinucleated giant cells, which have been described as being 'cannibalistic' because they often contain phagocytosed macrophages or neutrophils. Giant cell interstitial pneumonitis (GIP) is almost pathognomonic for hard metal disease [4, 5]. Similar multinucleated giant cells may be present in bronchoalveolar lavage (BAL) fluid, although BAL alone is probably not sufficient for diagnosis or exclusion of GIP [6]. Electron microscopy suggests that these giant cells may be either macrophages or type II alveolar epithelial cells in origin. These giant cells may represent a delayed-type hypersensitivity/cell-mediated immune response to cobalt.
This pathologic finding coupled with an appropriate clinical picture and an occupational history of exposure to cobalt makes the diagnosis of hard metal disease or cobalt-related interstitial lung disease. Lung biopsies of patients show fibrous tissue proliferation, metaplasia from squamous to cuboidal epithelium, mononuclear cells within alveoli (desquamative interstitial pneumonitis) and multinucleated giant cells [7, 8]. However, the differential diagnosis of this tissue reaction includes desquamative interstitial pneumonitis (DIP) and usual interstitial pneumonitis/idiopathic pulmonary fibrosis (UIP).
Question 3
Which ONE of the following occupations is classically associated with exposure to hard metal?
a) Aerospace industry
b) Diamond cutting and drilling
c) Auto body shop workers
d) Sandblasting
e) Construction
Answer
The correct answer is B.
Hard metal pulmonary disease was first recognized in metal dust workers in Germany in 1940. In 1977, based on observations in the National Occupational Health Surveys, NIOSH - The National Institute for Occupational Safety and Health estimated that there were approximately 30,000 workers in the United States potentially exposed to tungsten and cemented tungsten carbide [9]. Occupations associated with cobalt-related interstitial lung disease include those associated with the maintenance and resharpening of hard metal tools (commonly known as carbide-tipped tools), the production of hard metals, diamond tooling (grinding wheels and stone saws), work utilizing cemented tungsten carbide (oil well drilling sites), armored plate production (tanks, naval ships), and the manufacture and maintenance (grinding/polishing) of cutting tools and loops for fishing poles [10].
Much of the clinical experience with hard metal disease comes from studies of the diamond cutting and drilling industries, as well as from the tungsten carbide manufacturing industry. Cases have also been reported in the metal parts coating industry where hard metal is aerosolized under conditions of high temperature and pressure in a process used to coat tools, such as screw divers and chisels, with hard metal.
Question 4
Which ONE of the following is not a well-described presentation of lung disease following occupational exposure to hard metal?
(a) Occupational Asthma
b) Hypersensitivity Pneumonitis
c) Interstitial Fibrosis
d) Giant cell interstitial pneumonitis
e) Lung cancer
Answer
The correct answer is E.
In addition to interstitial fibrosis, occupational cobalt exposure may also cause occupational asthma, bronchitis, bronchiolitis obliterans-organizing pneumonia, hypersensitivity pneumonitis and acute chemical pneumonitis in the event of an acute high-level exposure. Cobalt is also a skin sensitizer, so skin hypersensitivity (as seen in this patient) and conjunctivitis may be seen. At this time, it is uncertain whether the risk of lung cancer is increased in hard metal workers.
Question 5
Which ONE of the following statements is FALSE regarding hard metal pulmonary disease?
a) Digital clubbing is seen in patients with advanced interstitial disease
b) Some susceptible individuals have a rapid progression of pulmonary fibrosis, which can lead to death.
c) Removal from exposure is beneficial; yet the disease may progress despite removal
d) Steroids are the mainstay of therapy
e) Even among exposed workers, the development of this disease is relatively rare
Answer
The correct answer is D.
Fortunately, this disease occurs rarely, even among exposed workers. Studies have shown that less than 1% of the exposed workers actually develop this disease [1]. The natural history of this disease is variable; if the patient presents early and is removed from dust exposure, there is an excellent chance for complete recovery. However, in later stages, progressive fibrosis with respiratory failure may develop [5, 11] even after cessation of exposure. Although no controlled clinical trials have been reported, the response to oral corticosteroids may be quite variable. Inhaled corticosteroids, cyclosporine, azathioprine, and cyclophosphamide have also been utilized.
Occupational hygiene is vital in preventing this disease. The current Occupational Safety & Health Administration or OSHA permissible exposure limit (PEL) for cobalt metal, dust, and fume in the United States is 0.1 milligram per cubic meter (mg/m3) of air as an 8-hour time-weighted average (TWA) concentration.