Craig Glazer, MD, MSPH
Assistant Professor of Medicine
University of Texas Southwestern Medical Center
Dallas, TX
AND
Elizabeth Barker
Program Coordinator
National Jewish Medical and Research Center
Denver, CO
AND
Lisa A. Maier, MD, MSPH
National Jewish Medical and Research Center
Associate Professor of Medicine University of Colorado School of Medicine
Denver, CO
A 44-year-old man, employed by a metal production company presents for a second opinion regarding his respiratory symptoms of cough and dyspnea on exertion. Both symptoms began in 1993, seven years prior to his current presentation, and have gradually progressed since that time. His cough is nonproductive. His dyspnea only occurs with exertion and has progressed. Initially, he only developed dyspnea with strenuous activity. Currently his dyspnea occurs with fast walking and is associated with chest tightness. He had a 27 pack-year history of tobacco use but quit 3 months prior to his presentation due to the progressive symptoms.
He works for a company that both mines and recycles copper and other metals. He worked in the mine without respiratory protection for 6 months in 1975 and for another 6 months in 1977. From 1978 to the time of presentation he worked at various jobs in the recycling portion of the plant. The recycling material comes from various sources including circuit boards and computers. His job duties included handling recycled metals which are sometimes pulverized, recovering slag in the smelter, and performing janitorial work including dry sweeping.
His past medical history is significant for two episodes of pneumonia. In addition, at the time of symptom onset in 1993 he had an abnormal chest radiograph with upper lobe predominant nodular disease. After a diagnostic evaluation, which excluded collagen vascular disease and vasculitis, he was diagnosed with chronic simple silicosis. He also participated in a whole lung lavage research protocol for silicosis. At the time of his lavage, the BAL cell differential revealed 63% lymphocytes.
On physical exam he is a thin man in no respiratory distress. He was afebrile and his vital signs were stable. The head and neck exam was benign. Lung exam revealed bilateral expiratory wheezing but no crackles. Heart exam revealed a regular rhythm with no murmurs gallops or rubs, or signs of cor pulmonale. Abdominal exam was benign, the extremities were without cyanosis, clubbing or edema and the neurologic exam was non-focal. A complete lymph node survey was also unremarkable.
His pulmonary function tests leading up to the current presentation revealed progressive obstructive lung disease:
Table 1: Spirometry for the patient discussed from 1989 until 1996
| PFT's | 7/89 | 5/90 | 2/92 | 2/93 | 5/94 | 3/96 |
| FVC | 5.95(104) | 6.17(106) | 5.17(89) | 5.32(88) | 4.94(83) | 5.47(100) |
| FEV1 | 3.69 (84) | 4.16 (87) | 3.56(74) | 3.36(61) | 2.92(59) | 2.96(66) |
| Ratio | 62 | 67 | 69 | 63 | 59 | 54 |
His current chest x-ray and high resolution CT scan are shown in figures 1a and 1b respectively.
Click on images to enlarge.
Figure 1a: Chest radiograph demonstrating upper lobe predominant nodular infiltrates, hilar adenopathy, and some apical pleura reaction
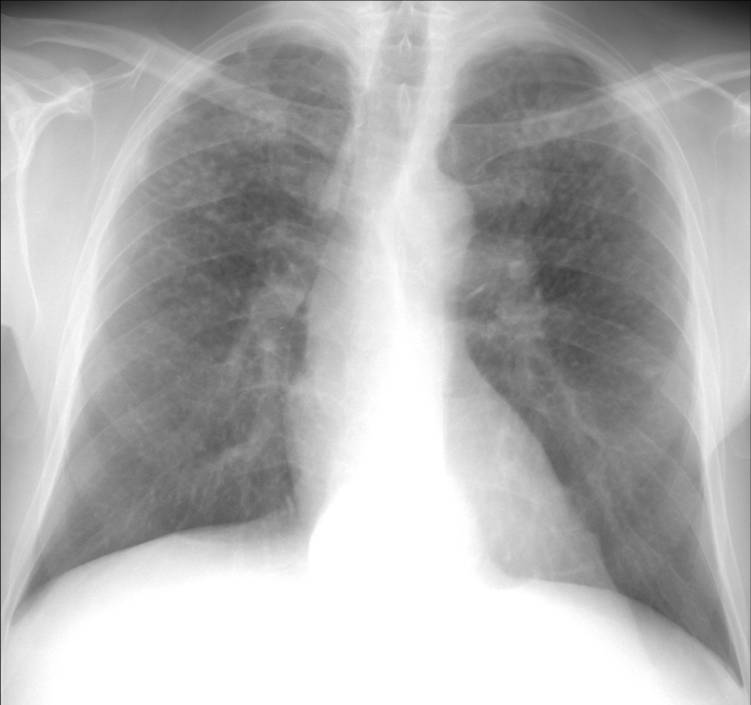
Figure 1b: High resolution chest CT scan through the upper lobes demonstrating bilateral nodular disease following the peribronchovascular distribution.

COMMENTS: Please send any comments to Akshay Sood, M.D., M.P.H.
SUGGESTED RESOURCES:
- National Jewish Medical and Research Center Website
- OSHA Website
- Department of Energy Current Worker Program Website
- Department of Labor/Energy Employees Illness Compensation Program Website
REFERENCES:
- Cullen, MR, Kominsky, JR, Rossman, MD, Cherniack, MG, Rankin, JA, Balmes, JR, Kern, JA, et al. Chronic beryllium disease in a precious metal refinery. Clinical epidemiologic and immunologic evidence for continuing risk from exposure to low level beryllium fume. Am Rev Respir Dis 1987;135(1):201-8.
- Newman, LS, Buschman, DL, Newell, JD, Jr., Lynch, DA. Beryllium disease: assessment with CT. Radiology 1994;190(3):835-40.
- Newman, LS, Maier, L. Beryllium. In: Sullivan J, Krieger G, eds. Clinical Environmental Health and Toxic Exposures. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001:919-26.
- Newman, L. Metals. In: Harber P, Schenker M, Balmes J, eds. Occupational and Environmental Respiratory Disease. St. Louis: Mosby; 1996:469-513.
- Kelleher, P, Pacheco, K, Newman, LS. Inorganic dust pneumonias: the metal-related parenchymal disorders. Environ Health Perspect 2000;108 Suppl 4:685-96.
- Rose, C. Hypersensitivity Pneumonitis. In: Murray JF NJ, ed. Textbook of Respiratory Medicine. 3 ed. Philadelphia: W.B. Saunders Company; 2000:1867-84.
- Glazer, CS, Rose, CS, Lynch, DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging 2002;17(4):261-72.
- Davis, G. Silica. In: Harber P, Schenker M, Balmes J, eds. Occupational and Environmental Respiratory Disease. St. Louis: Mosby; 1996:373-99.
- Health Effects of Occupational Exposure to Respirable Crystalline Silica. Cincinatti: Department of Health and Human Services; 2002.
- Cohen, C, Fireman, E, Ganor, E, Man, A, Ribak, J, Lerman, Y. Accelerated silicosis with mixed-dust pneumoconiosis in a hard-metal grinder. J Occup Environ Med 1999;41(6):480-5.
- Costabel, U, Bross, K, Huck, E, Guzman, J, Matthys, H. Lung and blood lymphocyte subsets in asbestosis and in mixed dust pneumoconiosis. Chest 1987;91(1):110-2.
- Mroz, MM, Kreiss, K, Lezotte, DC, Campbell, PA, Newman, LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J Allergy Clin Immunol 1991;88(1):54-60.
- Sawyer, RT, Maier, LA, Kittle, LA, Newman, LS. Chronic beryllium disease: a model interaction between innate and acquired immunity. Int Immunopharmacol 2002;2(2-3):249-61.
- Newman, LS. Significance of the Blood Beryllium Lymphocyte Proliferation Test. Environ Health Perspect 1996;104S(5):953-6.
- Newman, LS, Lloyd, J, Daniloff, E. The Natural History of Beryllium Sensitization and Chronic Beryllium Disease. Environ Health Perspect 1996;104S(5):937-43.
- Apostoli, P, Schaller KH. Urinary beryllium - a suitable tool for assessing occupational and environmental beryllium exposure ? Int Arch Occup Environ Health 2001; 74:162 - 166.
- OSHA. Preventing adverse health effects from exposure to beryllium in dental laboratories. HIB 02-04-19; 2002.
- Balkissoon, RC, Newman, LS. Beryllium copper alloy (2%) causes chronic beryllium disease. J Occup Environ Med 1999;41(4):304-8.
Question 1
In addition to silica, which of the following exposures could explain this patient's radiographic appearance/clinical presentation?
- Iron
- Copper
- Beryllium
- Cadmium
- Cobalt
Answer
The correct answer is C.
Of the metals listed, only beryllium could cause both this clinical picture and radiographic appearance with these exposures. Beryllium copper alloys are used in many high technologic and electronics applications including computers. Recycling computers to reclaim copper or other metals can lead to beryllium exposure in metal recycling workers(1). Chronic beryllium disease (CBD) is a granulomatous disease with slowly progressive respiratory symptoms caused by exposure and subsequent sensitization to beryllium. The typical HRCT appearance is similar to sarcoidosis and features primarily diffuse pulmonary nodules in a peribronchovascular and/or perilymphatic distribution. The pulmonary function abnormalities include obstruction, restriction, and mixed patterns (2, 3). In this case, the patient's cigarette abuse could have contributed to his obstruction but the lack of any emphysema on the CT scan makes that unlikely to be the sole explanation. Thus, this patient had potential for beryllium exposure during his work in metal recycling and has a disease course, radiographic, and physiologic presentation consistent with chronic beryllium disease.
Iron could cause a similar radiographic appearance (4). This condition is known as siderosis. However, siderosis is often considered a "benign" pneumoconiosis, as patients do not typically manifest either symptoms or pulmonary function abnormalities. Cadmium may cause emphysema or acute toxic pneumonitis but has not been reported to cause a nodular diffuse lung disease (5). Copper has only been reported to cause granulomatous lung disease in the rare instance of copper sulfate spraying as an herbicide in vineyards (hypersensitivity pneumonitis) (6). Therefore the exposure scenario doesn't apply and the radiographic pattern in this case is quite different from most descriptions of the radiology in hypersensitivity pneumonitis, which generally features diffuse centrilobular nodules of ground glass density, patchy ground glass opacities and air-trapping (7). Cobalt can cause hard-metal asthma and giant cell interstitial pneumonitis. Exposure to cobalt usually results from the manufacture and exposure to dusts from tungsten carbide tools or cobalt-diamond polishing disks (4), which this patient did not have.
Question 2
Which of the following factors about this case are atypical for chronic simple silicosis?
- A latency between onset of exposure and the appearance of disease of less than 20 years
- Short duration of exposure
- Worsening symptoms in the absence of progressive massive fibrosis
- Lymphocyte predominance on bronchoalveolar lavage
- B, C, and D
Answer
The correct answer is E.
Chronic simple silicosis usually has a latency of at least 10 years between exposure and disease onset, thus choice A is incorrect (8, 9). However, this patient's total exposure time in the mine area was actually brief and barring extremely high exposure levels during that time period it is unlikely his exposure would have lead to disease. Chronic simple silicosis is typically asymptomatic. Symptoms from silicosis usually occur with the development of progressive massive fibrosis, which this patient did not have. The one exception to this rule, is the presence of COPD, since patients exposed to silica dust are at increased risk of COPD (9). Our patient did have obstructive lung disease and this could have explained his symptoms, but again the exposure duration is short making COPD as a result of silica exposure less likely. The lymphocyte count on bronchoalveolar lavage may be elevated in silicosis but lymphocytes are not typically the predominant cell type (10, 11).
Based on the history of exposure to metals recovered from computers and other electronics beryllium exposure and subsequent chronic beryllium disease was suspected.
Question 3
Which of the following is the most appropriate next step in order to evaluate the possibility of chronic beryllium disease (CBD)?
- Peripheral blood beryllium lymphocyte proliferation test (BeLPT)
- Lung biopsy to obtain tissue for metal analysis
- Beryllium skin patch testing
- Fiberoptic bronchoscopy
- Urinary beryllium levels
Answer
The correct answer is A.
There are several published algorithms for the diagnosis of CBD, which center around the BeLPT (3). CBD occurs when the body develops a specific cell-mediated (TH1 type) immune response to beryllium. The response is beryllium antigen specific and associated with chronic, cytokine-medicated inflammation (12, 13). In an excellent example of translating basic research into clinical tools, researchers took advantage of this knowledge of pathogenesis to develop a test that can determine whether an individual's immune system recognizes beryllium. In the BeLPT, a patient's mononuclear cells are collected from peripheral blood or bronchoalveolar lavage and cultured in vitro in the presence and absence of beryllium salts. Cell proliferation is measured by the incorporation of tritiated thymidine into dividing cells (14). The beryllium specific cellular immune response is then quantified and reported as a "stimulation index": this is the ratio of the counts per minute of radioactivity in the cells stimulated by beryllium salts divided by the counts per minute for that person's cells that have not been stimulated with any beryllium (14).
The BeLPT is the most effective, sensitive and specific screening test currently available for the diagnosis of chronic beryllium disease in the clinic and in the workplace (3, 4, 15). The sensitivity of the BeLPT is between 80-90% and it is the first test one should obtain once beryllium disease is suspected. A patient is considered sensitized for clinical purposes if they have 2 positive BeLPTs . However, certain compensation systems, including the Department of Labor compensation program for Department of Energy workers, define beryllium sensitization on the basis of only one abnormal BeLPT. Once an individual is confirmed as sensitized, a bronchoscopy with bronchoalveolar lavage and transbronchial biopsy should be performed to evaluate and confirm diagnosis of beryllium disease.
Beryllium skin patch testing can also demonstrate immune system sensitization. However, it is not standardized and carries a theoretical risk of inducing sensitization. Skin testing is thus reserved for cases where the blood BeLPT is equivocal. Tissue analysis for beryllium is no longer recommended because it only proves exposure, not immunologic reactivity. One could also perform a bronchoscopy with BAL and perform the BeLPT on the lavage cells. However, as this is more invasive than the blood BeLPT the peripheral blood version is usually recommended as the first line test. However, negative results on the blood BeLPT do not rule out disease. In that setting, the test could either be repeated in different laboratories or if the clinical suspicion is high enough one should proceed with bronchoscopy and BAL as the sensitivity of the BAL BeLPT is about 95%. Urinary beryllium levels have been proposed as an assessment tool for monitoring recent beryllium exposure (16). Urinary beryllium levels serve as a marker of exposure but cannot discriminate workers with beryllium sensitization from those without sensitization.
The patient subsequently had 2 positive peripheral blood BeLPTs and he was diagnosed with beryllium sensitization. Further evaluation included a bronchoscopy to diagnose chronic beryllium disease and static and exercise pulmonary function testing for staging. His pulmonary function testing and exercise testing (shown below) reveal obstruction and abnormal gas exchange. His bronchoscopy revealed a lymphocytic alveolitis with 56% lymphocytes. A BeLPT was performed on the BAL and was positive with a peak stimulation index of 128 (normal <3). His transbronchial biopsies revealed non-necrotizing granulomas with negative stains and cultures for mycobacteria and fungi. There was no evidence for silicosis on the biopsies. Based on these results he was diagnosed with chronic beryllium disease.
Table 2: Pulmonary function testing revealing mild obstruction with air-trapping and no bronchodilator response. The DLCO was also moderately reduced.
| Lung Volumes | Pred | Baseline | % Pred | Post | % Pred | % Change |
| TLC (L) | 7.60 | 9.27 | 122 | 8.98 | 118 | -3 |
| TGV (L) | 4.28 | 5.55 | 130 | 4.93 | 115 | -11 |
| Forced Expiration | Pred | Baseline | % Pred | Post | % Pred | % Change |
| FVC (L) | 5.78 | 5.57 | 96 | 5.65 | 98 | 1 |
| FEV1 (L) | 4.25 | 3.33 | 78 | 3.47 | 82 | 4 |
| FEV1/FVC (%) | 73 | 60 | 82 | 61 | 84 | 3 |
| Additional Studies | Pred | Baseline | % Pred | Post | % Pred | % Change |
| DLCO | 41.16 | 26.09 | 63 | |||
| VA (L) | 7.37 | 7.65 | 104 | |||
| DLCO/VA (ml/min/mmHg/L) |
5.58 | 3.41 | 61 |
Table 3: Cardiopulmonary exercise test results.
The test was performed with an indwelling arterial catheter. The patient had a normal total workload and cardiac response. However, his oxygenation worsened significantly during exercise with a drop in his PaO2 from 74 to 50 and an increase in his alveolar-arterial gradient from 10.1 to 39.9. His ventilatory response was abnormal with an increased respiratory rate and elevated Vd/Vt at rest and with exertion.
| Predicted | Baseline | Max. Exer | % Predicted | |
| Metabolics: | ||||
| Work Load (Watts) | 252 | 240 | 95 | |
| VO2 (L/min) | 3.249 | 0.271 | 2.289 | 70 |
| VCO2(L/min) | 0.260 | 2.611 | ||
| RQ | 0.96 | 1.14 | ||
| Cardiovascular: | ||||
| HR (BPM) | 5.78 | 78 | 164 | 91 |
| O2 Pulse (mL/Beat) | 4.25 | 3.5 | 14.0 | 78 |
| Systolic BP (mmHg) | 73 | 130 | 175 | 80 |
| Diastolic BP (mmHg) | 80 | 91 | ||
| Ventilation: | ||||
| VE-BTPS (L/min) | 41.16 | 18.9 | 119.2 | 88 |
| RR (BPM) | 25 | 53 | ||
| VD/VT-ABG (%) | 0.53 | 0.32 | ||
| VT (Liters) | 41.16 | 0.740 | 2.257 | 66 |
| Gas Exchange: | ||||
| FlO2 (%) | 20.61 | 20.63 | ||
| SpO2 (%) | 93 | 84 | ||
| SaO2 (%) | 94 | 83 | ||
| PH | 7.44 | 7.34 | ||
| PaCO2 (mmHg) | 34.0 | 34.0 | ||
| PaO2 (mmHg) | 74.0 | 50.0 | ||
| HCO3-(meq/L) | 23.7 | 18.3 | ||
| A-aDO2 (mmHg) | 10.1 | 39.9 | ||
| Lactate (mmole/L) | 0.6 | 5.3 |
Question 4
Treatment could reasonably include which of the following?
- Minimize further exposure
- Inhaled bronchodilators
- Inhaled steroids
- Systemic oral corticosteroids
- All of the above
Answer
The correct answer is E.
Additional testing to evaluate the extent of disease involvement or level of impairment may include a full set of pulmonary function tests with DLCO and an assessment of exercise tolerance and oxygenation with exercise, as obtained on this patient. Medications are then instituted if impairment in pulmonary function is present. There have been no controlled trials of therapy in CBD. Minimizing further exposure is considered medically prudent and usually recommended. We generally recommend minimizing further exposure at the time an immune response to beryllium is demonstrated that is with a diagnosis of beryllium sensitivity, regardless of the presence or absence of symptoms, pulmonary function or pulmonary parenchymal (either on transbronchial biopsy or HRCT) abnormalities. The standard approach to therapy is to initiate treatment with systemic corticosteroids for symptomatic or progressive pulmonary function abnormalities, such as noted in this case. The goal of treatment is reversal or stabilization of the inflammatory response to beryllium. The starting dose is usually 40 mg of prednisone either daily or every other day. This is then titrated down to the minimal effective dose over the next 3 to 6 months. Other supportive therapies include exercise, immunizations (pneumococcal vaccine every 5-7 years and yearly influenza vaccine), and oxygen if resting or exercise induced hypoxia develop. Other symptomatic therapies should include diuretics and other treatment for cor pulmonale. We have also found that many patients have airway involvement with symptoms of cough and wheeze. Many of these patients note improvement in these symptoms with inhaled steroids and/or bronchodilators. Thus, treatment could reasonably include all of the above options.
Question 5
In addition to metal recycling, workers in which of the following industries have potential for beryllium exposure?
- Aerospace
- Nuclear weapons production
- Dentistry
- Ceramics
- All of the above
Answer
The correct answer is E.
Acute and chronic beryllium disease was first described in the fluorescent light industry. Beryllium is no longer used in that application; however, due to many advantageous properties, its range of uses has widely spread. Current estimates indicate that at least eight hundred thousand U.S. workers are exposed or have been exposed to beryllium in the past. During the cold war the primary use of beryllium was in the nuclear weapons industry. Presently, beryllium is used in a variety of applications including electronics, computers, high technology, ceramics, non-sparking tools, the aerospace industry, and even the dental industry in the production of dental prosthesis (17). Beryllium is used in its pure form, as oxides for ceramics, and in various alloys, especially beryllium copper. Beryllium disease can occur in workers exposed to beryllium in any of these forms (18). The majority of workers with beryllium sensitization and disease are exposed to beryllium alloys. Individuals who use beryllium containing items without producing a respirable particulate are probably at minimal risk of developing disease. Those that produce a respirable particulate, such as production workers, machinists or recyclers, or who disturb or are exposed to bystander beryllium dust and as maintenance workers or administrative workers are at risk of disease. Importantly, due to the involvement of adoptive immunity in the pathogenesis of the disease the dose response is not linear and large levels of exposure are not required. Therefore, physicians must maintain a high degree of clinical suspicion when evaluating patients with a history of direct or indirect exposure to beryllium, beryllium oxides or metal alloys containing beryllium.



