By Ellen L. Burnham, MD and Marc Moss, MD
The application of findings derived in basic science to the development of new understanding of disease mechanisms, diagnoses, and therapeutics in humans is known as translational research [1]. More generally speaking, it is the movement of ideas from the basic science laboratory to the clinical arena. Although the number of investigators performing patient-oriented translational research is small, these individuals are vital to the future of medicine. Over the past several years the importance of this type of research has been increasingly recognized. Assistance with some of the difficult tasks faced by translational investigators appears to be more forthcoming in recent years than in the past, as will be discussed in this monograph. The concept of heterogeneity and its relationship to clinical research will also be discussed.
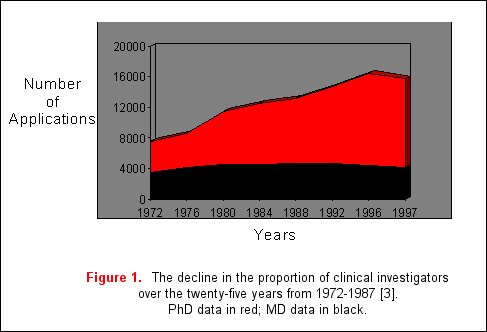
With the development of diverse new technologies, remarkable advances have occurred in the understanding of the molecular and genetic bases of disease. As a result, basic science knowledge has outstripped progress in clinical research. A major reason for this includes reduced financial security of medical centers, forcing a shift in the focus of clinicians towards patient service, and away from research. Additionally, patient-oriented research applications in NIH study sections receive poorer priority scores and funding rates when compared to basic science research applications, especially when these applications are assigned to study sections unfamiliar with reviewing clinical science [2]. Furthermore, in academic medical centers, clinical research careers are generally regarded as less prestigious than basic science careers [1]. As a result, the proportion of physician scientists who are performing clinical research has declined from 40% in 1972 to 25% in 1996 with a proportionate increase in the number of basic science investigators (figure 1) [3]. This data is based on the assumption that a majority of clinical researchers will hold MD degrees, in contrast to PhDs who are more likely to engage in basic science research. Due to these concerns, the Institute of Medicine launched a study of this problem in 1993 to identify the number of NIH applications devoted to translational research and reported that only 10% of the NIH extramural budget was devoted to translational research [4].
Translational research may be considered a unidirectional flow of ideas, or a "bridge" from basic science to clinical application, based on the pharmaceutical model of drug development. Initially, studies are necessary to determine whether findings in animal models actually apply to true human disease states. From there potential therapies need to be identified and developed, and then undergo the rigorous methodologies of phase I, II, and III trials. In this model, epidemiological studies that capitalize on heterogeneity in a population identify clinically important associations, fueling basic science investigation and further clinical translational research. Another model for translational research is bi-directional, where ideas flow from the bedside to the bench and back again.
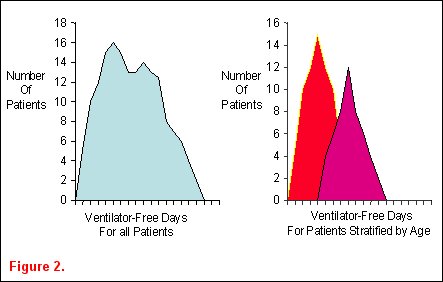
Ideas for epidemiological studies in an attempt to identify important associations between demographic characteristics and clinically relevant outcomes often arise from observing ICU patients and reading the medical literature. Heterogeneity in patient populations is a key concept in identifying clinically relevant associations that warrant further investigation. Classically, epidemiological studies describe the distribution of patient characteristics in order to establish associations between these factors and human disease. They are often purely descriptive and useful in public health issues or resource allocation. Epidemiological studies can also be analytical, where determinants of variability in a disease are sought. Biological plausibility for hypotheses in these types of epidemiological studies arises from critical reading of a publication or careful data examination. For example, an investigator examines the distribution of ventilator-free days among the cohort of patients enrolled into the ARDSnet ventilator trial and finds it is not normally distributed (figure 2a) Upon stratification of the patients into two age groups, the heterogeneity in the distribution appears to be due to the age of the patients (figure 2b) [5].
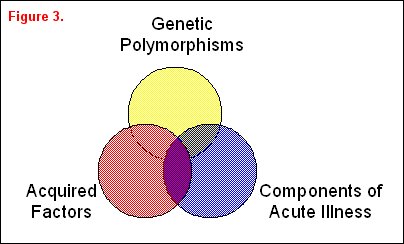
A variety of risk factors may be associated with or even responsible for differences in a clinically important outcome variable that can be arbitrarily divided into three overlapping groups: genetic factors, acquired factors, and components associated with acute illness (figure 3). A classic example of genetic factors affecting outcome was illustrated by Sorensen and colleagues [6], who studied causes of premature death in children who were adopted at an early age. Both the children's biological and adoptive parents were identified. The relative risk of death from a variety of causes was increased in the adopted children whose biological parents had died at an early age, thus suggesting a potential genetic etiology of the increased risk of premature death. In a more recent genetic study, Marshall reported the increased frequency of a polymorphism in the ACE gene in patients with ARDS, suggesting that a genetic variant can increase the likelihood of developing ARDS [7]. Moss and colleagues have reported that a prior history of alcohol abuse, an acquired risk factor, is associated with an increased incidence and severity of ARDS [8]. Work by Nuckton and colleagues highlights an example of a component of acute illness correlating with outcome [9]. They identified risk groups at risk for high ARDS mortality based on the dead space fraction, a component of this acute illness. In this study ARDS mortality increased in a "dose-dependent" fashion with the dead-space fraction. Quality of care is another component associated with acute illness. For example, whether or not a consultant intensivist supervises patient care in the ICU has an impact on both ICU and overall hospital mortality [10].
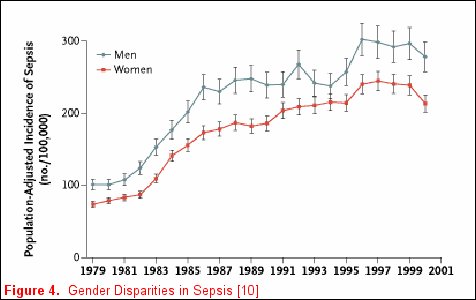
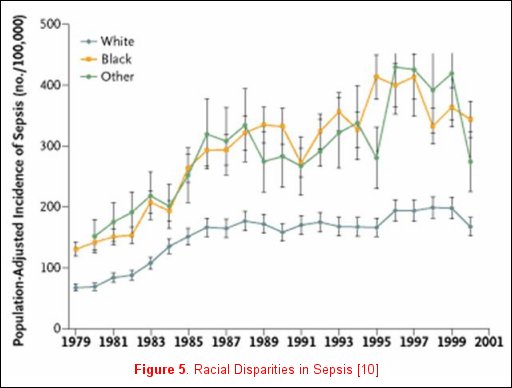
Unfortunately, not all risk factors for clinically relevant outcomes fit into a nice package, as no factor is purely genetic, acquired, or related to the acute illness. For example, Martin and colleagues reported clinically relevant associations that do not neatly fall inside any one of these categorizations [11] utilizing the National Hospital Discharge Survey that accounts for 750 million hospitalizations over a 22-year period. When the data were examined and patients where stratified by gender, men consistently had a higher incidence of sepsis (figure 4). Moreover, when the data were stratified by race, African-Americans and individuals of other races had a consistently higher incidence of sepsis (figure 5). The etiology of these racial and gender disparities is probably related to genetic factors, acquired factors, and components of the acute illness. Future studies are necessary to further explore these associations. It is important to realize that factors associated with disease development may not be associated with disease outcomes. In Nuckton's study [9], the size of the dead space ventilation, although associated with mortality, is unlikely to be a risk factor for the development of ARDS.
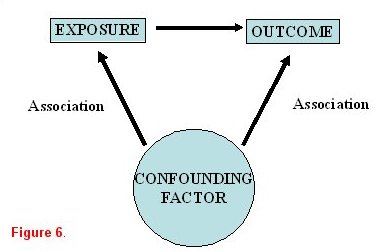
When studying an exposure-disease relationship, it is imperative to determine if the association is causal in nature. Observed associations may actually be (either completely or in part) due to one or more other factors that had been unrecognized. These are known as confounders. Therefore, once an association has been identified in a univariate analysis, it is important to confirm its effects in a stratified or multivariable analysis to account for possible confounders or effect modification. As illustrated in figure 6, an exposure is identified that is believed to be associated with a specific outcome. A confounding variable is a third factor that is associated with both the exposure and the outcome.
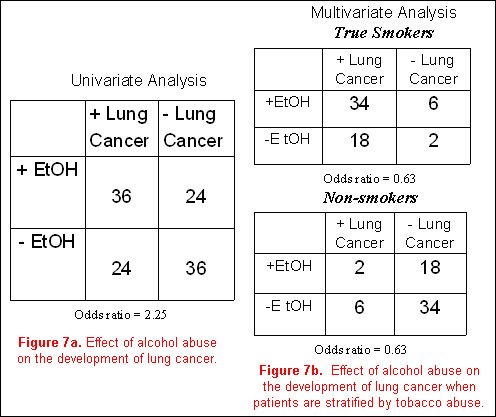
Using a classic epidemiological example, it is believed that alcohol abuse (the exposure) is related to the development of lung cancer (the outcome). Our confounding variable of concern is smoking. To be a true confounder, smoking must be associated with alcohol abuse, and must also be associated with lung cancer. Additionally, in a stratified analysis accounting for the effects of the confounder (smoking), the association between alcohol and lung cancer should be eliminated.
In a hypothetical model, a cohort of subjects with a positive history of alcohol abuse is more likely to develop lung cancer than non- drinkers, with an odds ratio of 2.25 (figure 7a). However, analyzing the entire cohort after first stratifying subjects into smokers and non-smokers, and then examining the effects of alcohol on the incidence of lung cancer in each of these two groups separately, one finds that the effects of alcohol abuse on the development of lung cancer go away (odds ratio for both analyses is 0.63, (figure 7b). Obviously, many more cases of lung cancer are present in the smoking individuals compared to the non-smokers. The initial concept that alcohol abuse is associated with lung cancer is not substantiated, and the association was completely due to the effects of the confounding variable (in this case, smoking).
Potential confounding variables can be identified by careful examination of the literature. When something has been reported previously to be an important risk factor for the outcome of interest (such as smoking for lung cancer), it needs to be examined as a confounding variable. With large samples (databases), some factors such as race, gender, and age should be examined as potential confounding variables. Finally, avoid including variables in the causal pathway as potential confounders as they will mask risk factors that may be causal in a given disease state. For example, if regular physical activity is being examined in relationship to cardiovascular disease, one might not include HDL levels in the multivariable analysis because the effect of exercise on HDL levels may be the mechanism by which physical activity limits the cardiovascular disease, thus masking the effects of a sedentary life style on cardiovascular disease.
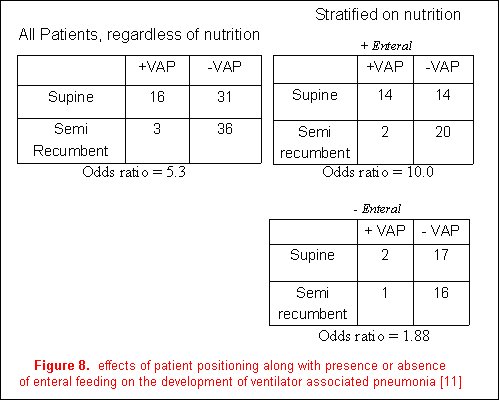
Effect modification describes the variation in association between exposure and disease according to the magnitude of another factor. An example from the recent literature is the relationship between body position (the primary exposure) and the incidence of ventilator-associated pneumonia (the outcome variable) [12]. The authors reported a decreased frequency of ventilator-associated pneumonia in a semi-recumbent patient position (8%) than in a supine position (34%); however, the presence of enteral feedings was demonstrated to be an effect modifier. The magnitude of the effect of body position on the development of VAP was stratified based on the presence or absence of enteral feeding. Although the association of patient positioning and the development of ventilator-associated pneumonia were still evident in patients not receiving enteral nutrition, it is much greater in those who are (figure 8). It is therefore more accurate to discuss the effects of body position on VAP in relation to the presence or absence of enteral feeding as it is an effect modifier on the relationship between body position and the development of VAP.
The next step in examining a hypothesis is to determine how significant an association may be using Bradford-Hill criteria. These criteria highlight aspects of an association that should be evaluated before deciding whether an association is causal, and include the strength of an observed association, the consistency of the association, and the temporality of the association (cause precedes effect in time). A dose-response effect may be observed between the causal agent and the outcome, and there should also be biologic plausibility for the association, with a specific cause-and-effect relationship. The stronger an association, the more likely it is to be causal, and associations that are consistently reported in the literature are more likely to be causal.
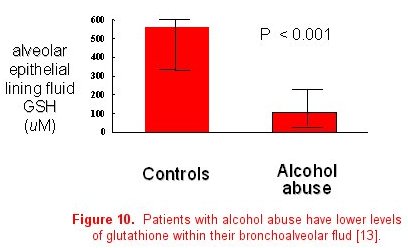
The importance of collaboration between basic science and clinical researchers to determine the mechanisms responsible for epidemiological associations cannot be underestimated. Once a clinically important association has been identified, translation to the basic science laboratory is often necessary to determine the actual causal pathway or mechanism. For example, basic science investigators in our group developed a septic model to examine the effects of alcohol abuse in rats. They determined that chronic ethanol ingestion in rats renders the lung susceptible to endotoxin-mediated edematous injury (figure 9) [13]. This was consistent with our clinical epidemiological studies. Glutathione deficiency was proposed as a mechanism to account for these findings through its effects on surfactant secretion, apoptosis, and alveolar epithelial fluid transport, leading to increased susceptibility for ARDS. These investigators examined the first part of this proposed mechanism in the animal model, reporting that ethanol-fed rats have significantly decreased glutathione concentrations in lung tissue, lung lavage fluid, and alveolar type II cells. Finally, glutathione replacement therapy decreased lung weight gain in an in vivo model of endotoxin-mediated lung injury and decreased protein concentrations in bronchoalveolar lavage fluid, thereby establishing a causal relationship between ethanol-induced glutathione depletion and acute lung injury. To further demonstrate the bi-directional flow of information, the third step is to determine if the basic observations and therapies are applicable to humans. In 2000, we reported that individuals with a positive history of chronic alcohol abuse have decreased concentrations of GSH in the epithelial lining fluid of the lung, similar to what was observed in the animal model (figure 10) [14].
In summary, heterogeneity in the incidence of ARDS due to chronic alcohol abuse was initially observed in a clinical sample of patients with ARDS. This led to further examination of the mechanisms that could cause the discrepancies between alcoholics and non-alcoholics, involving basic science models for mechanistic investigations. Ultimately, discoveries made at the bench were examined prospectively in a cohort of patients where mechanisms operative in animals were determined to be applicable to human subjects. The door is now open to explore therapies for ARDS in the cohort of patients with chronic alcohol abuse (about 50% of patients with ALI in our medical system).
Heterogeneity may be a beneficial phenomenon for identifying specific subgroups of patients that merit further investigation in a particular disease state. However heterogeneity may be problematic when evaluating specific therapies for ICU patients. The ICU population comprises a heterogeneous group of patients with syndromes (eg ARDS, sepsis) rather than diseases, leading to difficulties in the performance of clinical trials in the ICU. Patient heterogeneity may account for some of the disappointing results of clinical trials for ICU patients, as some therapies may only benefit a specific sub-group of ICU patients. Examples include activated protein C for severely septic patients, or a low tidal volume ventilation strategy for ARDS patients. Other therapies that may benefit a larger proportion of ICU patients include conservative transfusion strategies, as well as weaning and sedation protocols.
Some investigators have recommended new definitions of ICU syndromes based on biochemical criteria of inflammation, rather than clinical parameters to direct therapies at specific inflammatory components. These definitions would enable identification of more homogeneous patient populations that could respond to a specific therapy more predictably and beneficially. As an example, glutathione replacement therapy may only work for ARDS patients with pre-existing glutathione deficiency, such as in a patient with a history of chronic alcohol abuse. Similarly, anti-TNF antibodies may only be benefit septic patients who are TNF hypersecretors based on genetic polymorphisms, and might be harmful for those septic patients who are TNF hyposecretors. The potential of these therapies to benefit a particular type of ICU patient may be masked if the therapy is studied in a heterogeneous patient population.
Performance of successful translational research is highly dependent on an investigator's environment, as evidenced by the examples cited in this monograph. Collaboration between basic science and clinical investigators is essential with team-based research as a model. Training of future translational scientists will require a broad-based curriculum that includes molecular biology and genetic techniques, epidemiology and biostatistics, and clinical trial design and implementation . Several recommendations of the Director's Panel on Clinical Research hoping to improve the quality and quantity of translational research have been proposed, including increased training opportunities for investigators. These include the new K series awards for clinical research, special emphasis study sections to review clinical research, the institution of educational debt relief programs for clinical investigators, and the symbolic renaming of the SCCOR grants from Specialized Centers of Research to Specialized Clinical Centers of Research [15]. Additionally, there has been increased funding and visibility of general clinical research centers. Over 70 such federally-funded facilities currently exist across the US that provide inpatient and outpatient facilities and several core laboratory facilities. This establishes an infrastructure to conduct safe, state-of-the-art clinical research.
In conclusion, epidemiological studies can identify clinically important associations between demographics, risk factors, and outcome by examining heterogeneity of patient populations. The discovery of mechanisms underlying these associations may lead to goal directed therapies that will ultimately improve the outcome of the critically ill patients we care for on a daily basis.
References:
- Nathan DG. Careers in translational clinical research - Historical perspectives, future challenges. JAMA 2002;287(18):2424-7.
- Williams GH, Wara DW, Carbone P. Funding for patient-oriented research. Critical strain on a fundamental linchpin. JAMA 1997; 278(3):227-31.
- Nathan DG. Clinical research: perceptions, reality, and proposed solutions. National Institutes of Health Director's Panel on Clinical Research. JAMA 1998; 280(16):1427-31.
- Kelley WN, Randolph MA, eds. Careers in Clinical Research: Obstacles and Opportunities. Washington, DC: National Academy Press; 1994.
- Ely EW. Wheeler AP. Thompson BT. Ancukiewicz M. Steinberg KP. Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med 2002;136(1):25-42..
- Sorensen TI. Nielsen GG. Andersen PK. Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. New Eng J Med 1988; 318(12):727-32.
- Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ, Humphries SE, Hill MR, and Laurent G. Angiotensin Converting Enzyme Insertion/Deletion Polymorphism Is Associated with Susceptibility and Outcome in Acute Respiratory Distress Syndrome Am. J. Respir. Crit. Care Med 2002;166: 646-650.
- Moss M. Bucher B. Moore FA. Moore EE. Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996; 275(1):50-4.
- Nuckton TJ. Alonso JA. Kallet RH. Daniel BM. Pittet JF. Eisner MD. Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. New Eng J Med 2002; 346(17):1281-6.
- Manthous CA. Amoateng-Adjepong Y. al-Kharrat T. Jacob B. Alnuaimat HM. Chatila W. Hall JB. Effects of a medical intensivist on patient care in a community teaching hospital. Mayo Clin Proc 1997;72:391-399.
- Martin GS. Mannino DM. Eaton S. Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New Eng J Med 2003; 348(16):1546-54.
- Drakulovic MB. Torres A. Bauer TT. Nicolas JM. Nogue S. Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 1999;354(9193):1851-8.
- Holguin F. Moss I. Brown LA. Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998; 101(4):761-8.
- Moss M. Guidot DM. Wong-Lambertina M. Ten Hoor T. Perez RL. Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000; 161(2 Pt 1):414-9.
- The NIH Director's Panel on Clinical Research. Report to the advisory committee to the NIH director. December 1997.