Pun About Bone
Mohammed Megri, MD
3rd year Pulmonary and Critical Care fellow
Internal Medicine Department, Pulmonary and Critical Care Division, University of Kentucky
Case:
A 65-year-old non-smoking Caucasian female presented with 2-years of progressively worsening dyspnea and dry cough. She denied any history of malignancy, sick contacts, pets, or significant exposures. On physical examination, she was noted to have scattered crackles. Sputum culture and viral respiratory panel were negative, and autoimmune serology workup, including ANA, RF, MPO, PR-3, Anti-CCP, C-ANCA, P-ANCA, and anti-Scl-70, were within normal limits. Chest x ray, CT chest revealed the below findings (Figure 1):
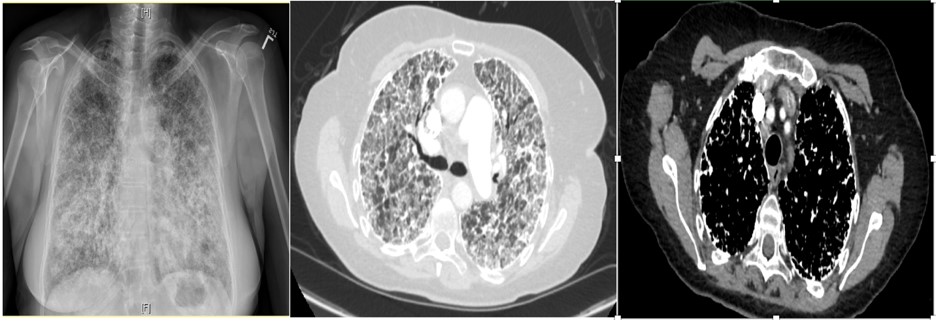
Figure 1. PA chest x-ray (left), Axial plane CT chest with contrast lung and mediastinal window
Question:
What is the least invasive approach in confirming the diagnosis?
- Bronchoscopy with bronchoalveolar lavage
- Induced Sputum
- Bronchoscopy with transbronchial biopsy
- High resolution computed tomography (HRCT)
- Pulmonary function testing
B. Induced Sputum
Discussion:
The patient has pulmonary alveolar microlithiasis (PAM), which manifests as progressively worsening dyspnea and dry cough. PAM is a diffuse intra-alveolar accumulation of spherical calcium phosphate concentrations. There is no sex predilection. It is hypothesized to be an autosomal recessive disease from a mutation of the solute carrier family 34 (sodium phosphate) member 2 gene (the SLC34A2)1. This gene is expressed only in alveolar type II cells. The mutation leads to the deposition of calcium phosphate2.
Pulmonary calcification is subdivided into idiopathic (pulmonary alveolar microlithiasis), metastatic (benign etiologies include end stage renal disease, primary hyperparathyroidism, hypervitaminosis D, Paget’s disease; this can also arise in the setting of y related hypercalcemia), and dystrophic (granulomatous disorders, infections, silicosis, coal worker pneumoconiosis)3.
Diagnosis of PAM can be confirmed non-invasively by the presence of microlithiasis in induced sputum in the absence of any of the other conditions noted above (metastatic or dystrophic) or clinical history of relevant exposures. On chest X-ray and computed tomography, PAM has a characteristic appearance of bilateral sand-like micronodular densities that obliterate the diaphragm and mediastinal border (Figure 2). Other imaging studies can be used as a screening tool because they are generally nonspecific for most types of pulmonary calcifications. HRCT mediastinal window can help to detect classifications (Figure 3) and it is more sensitive and specific than chest radiograph4, but it is not enough to confirm the diagnosis of PAM and be able to differentiate between the other causes of pulmonary calcifications (D wrong answer)4. Of the imaging studies, positive pulmonary bone scintigraphy with bone-avid radiotracer, 99mTc-MDP (Figure 4) can be used to confirm diagnosis. PFT may be normal at the beginning of the disease course and later show a restrictive pattern, which can be used to identify and monitor pulmonary impairment (E wrong answer). Because the presence of microlithiasis in the sputum is enough to confirm the diagnosis of PAM, invasive procedures such as bronchoscopy with BAL and transbronchial biopsy is usually not needed for diagnosis (A and C wrong answer). However, if the induced sputum is not diagnostic then bronchoscopy with BAL is required to confirm the diagnosis2. PAM treatment is supportive with oxygen supplementation if the patient is hypoxic, but the main intervention in those with severe disease is lung transplantation.
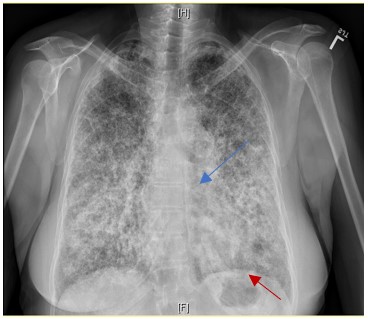
Figure 2. Sand-like micronodular densities obliterate the diaphragm (red arrow) and mediastinal (blue arrow) border
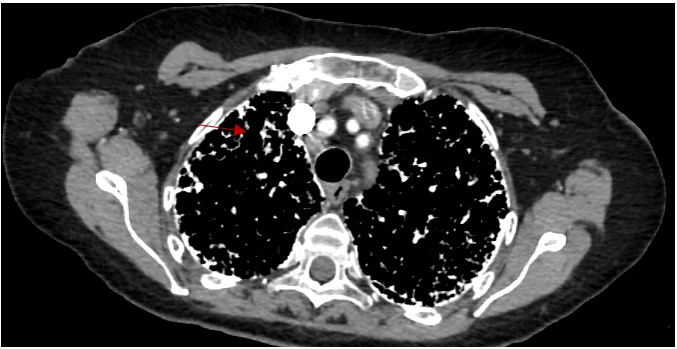
Figure 3. HRCT mediastinal window shows the micronodular calcifications
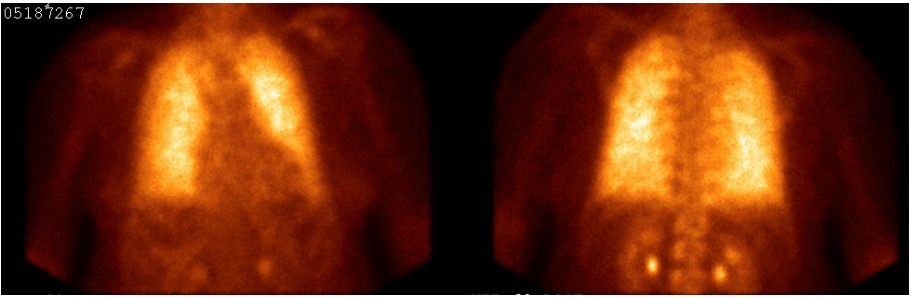
Figure 4. Positive pulmonary bone scintigraphy with bone-avid radiotracer, 99mTc-MDP positive for PAM
References
-
Corut A, Senyigit A, Ugur SA, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet 2006;79(4):650–656.
-
Castellana G, Castellana G, Gentile M, Castellana R, Resta O. Pulmonary alveolar microlithiasis: Review of the 1022 cases reported worldwide. Eur Respir Rev [Internet] 2015;24(138):607–620. Available from: http://dx.doi.org/10.1183/16000617.0036-2015
-
Ferreira Francisco FA, Pereira E Silva JL, Hochhegger B, Zanetti G, Marchiori E. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir Med 2013;107(1):1–9.
-
Siddiqui NA, Fuhrman CR. Best cases from the AFIP: Pulmonary alveolar microlithiasis. Radiographics 2011;31(2):585–590.



