TB or Not TB
1Frances Flanagan, MB, BCh, BAO, 1Lana Mukharesh, MD, 1Kenan Haver, MD
1Division of Pulmonary Medicine, Department of Pediatrics, Boston Children’s Hospital,
and Harvard Medical School, Boston MA.
Case
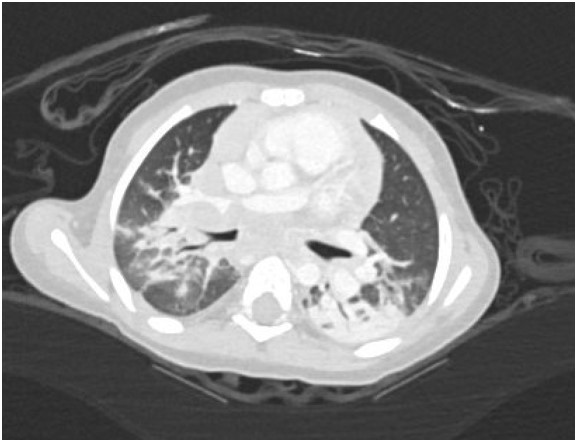
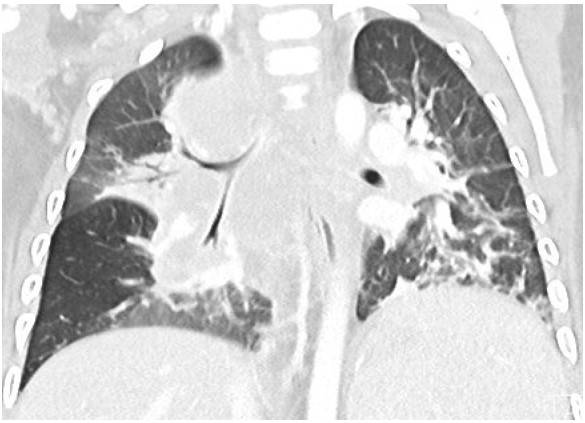
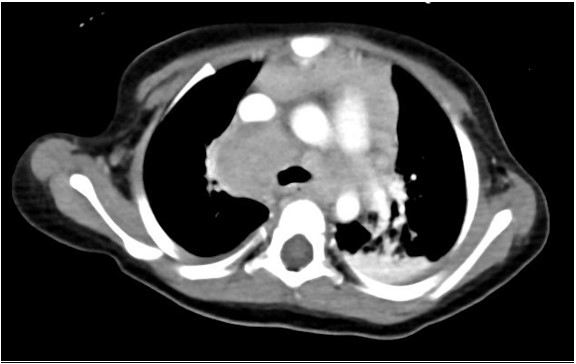
A 17-month-old previously healthy girl presented with a 6-week history of fever, poor appetite and fatigue. She had no exposure or travel history of note. An initial evaluation during week 1 of her illness included a normal physical examination, a CXR that was interpreted as normal, negative blood and urine cultures, a normal echocardiogram but elevated inflammatory markers (ESR 50mm/hr, CRP 5.3mg/dL). Due to continued fevers, weight loss of 1lb during her illness, persistently elevated inflammatory markers and a negative tuberculin skin test (TST), a repeat CXR was undertaken. Due to concerning features of hilar and paratracheal lymphadenopathy, a chest CT scan was subsequently performed (see images 1-3 below).
Question
The CT findings are most suggestive of infection due to:
- Aspergillus fumigates
- Human metapneumovirus
- Mycobacterium flavescens
- Streptococcus pneumoniae
- Mycobacterium tuberculosis
C. Mycobacterium flavescens
Discussion
The chest CT reveals a large conglomerate of diffuse bulky hilar and mediastinal non-calcified lymphadenopathy, with mild neck adenopathy, bilateral mainstem airway compression, scattered atelectasis and a post obstructive left lower lobe pneumonia. These findings are most consistent with an infection due to non-tuberculous Mycobacterium (NTM). It is notoriously difficult to differentiate TB from NTM on imaging in children and hence the need for laboratory organism confirmation. This is in comparison to adult patients where NTM are more commonly associated with bronchiectasis than TB and patients with TB tend to have more cavitary lesions, nodules and pleural effusions than those with NTM1. Human metapneumovirus infections typically appear with ill-defined patchy consolidation and may have mediastinal lymph node enlargement but not typically the large conglomerate of diffuse bulky lymphadenopathy seen on this child’s CT. Findings on CT in children with Aspergillus fumigates most often include segmental and multilobar consolidation, peripheral infiltrates, multiple small nodules, and larger peripheral nodular masses2. Bacterial infection in the lungs, including from Streptococcus pneumoniae, typically present with alveolar consolidation in a lobar and segmental distribution3.An extensive work up for malignancy, autoinflammatory conditions and infectious processes was undertaken. She had a negative TST and an Interferon-γ release assay (IGRA) was not performed due to its reduced sensitivity in children under 2 years of age compared to TST4. A cervical lymph node biopsy returned positive on day 18 for acid fast bacilli (AFB). Subsequently one of three gastric aspirates was found to have acid-fast bacilli.
She was placed on empiric tuberculosis therapy (rifampicin, isoniazid, pyrazinamide & ethambutol), whilst awaiting further speciation. Simultaneously she underwent immunological testing which showed compound heterozygous variants in the IFNGR1 gene (Interferon Gamma Receptor 1 ) (C.523del, p.Tyr175Metfs*2 (frameshift), heterozygous and C.200+1G>A (splice site) heterozygous), which have been reported with disseminated mycobacterial infections. This diagnosis was supported by normal lymphocyte counts but no expression of CD119/ IFN-γR on monocytes, and no activation of IFN-γ stimulated by pSTAT in monocytes.
IFN-γR1 deficiency is caused by a heterozygous or homozygous loss of function mutation in IFNGR1, leading to immunodeficiency 27A (autosomal recessive) or 27B (autosomal dominant). 40 unique mutations in IFNGR1 have been reported to date5. IFN-γR1 deficiency amongst other immunodeficiencies affecting IFN-γ production and/or signaling are often designated as Mendelian susceptibility to mycobacterial disease (MSMD)6. IFN-γ plays a key role in innate immunity against mycobacteria (via the type 1 cytokine pathway), and hence the hallmark feature of IFN-γR1 deficiency is disseminated disease with weakly virulent mycobacteria (e.g. environmental non tuberculous mycobacteria) and the live, attenuated Mycobacterium bovis bacille calmette-guerin (BCG) strain that is used in the vaccine against tuberculosis7. Of note TSTs use purified protein derivative (PPD) as the test reagent, which is a heterogeneous mixture of more than 200 mycobacterial peptides, some of which are expressed by both Mycobacterium tuberculosis and NTM. Studies report a positive TST in only 30-60% of children with NTM infections, whereas IGRAs are specifically designed to detect infection with Mycobacterium tuberculosis and thus usually negative in NTM8. Infections with other pathogens such as Salmonella, Histoplasma, Toxoplasma and viruses are occasionally found in patients with IFN-γR1 deficiency7. Four case reports to date have also linked IFN-γR1 to malignancies including Kaposi sarcoma, pineal germinoma and B cell lymphomas5.
Ultimately her culture returned with an isolate with 99% similarity to Mycobacterium flavescens. Thereafter her antimicrobial therapy was adjusted, based on sensitivities, to azithromycin, linezolid, ciprofloxacin and continuation of rifampicin. She has responded well to therapy and is currently asymptomatic with resolution of her leukocytosis and normalization of inflammatory markers. A follow up chest x-ray showed resolution of her previously noted adenopathy and was otherwise grossly normal. Definitive treatment for mendelian susceptibility to mycobacterial disease (due to IFNGR1 heterozygosity) is a hemopoietic stem cell transplant, which is planned for the near future9.
References
-
Yuan MK, Chang CY, Tsai PH, Lee YM, Huang JW, Chang SC. Comparative chest computed tomography findings of non-tuberculous mycobacterial lung diseases and pulmonary tuberculosis in patients with acid fast bacilli smear-positive sputum. BMC Pulm Med. 2014;14(1). doi:10.1186/1471-2466-14-65
-
Ankrah AO, Sathekge MM, Dierckx RAJO, Glaudemans AWJM. Imaging fungal infections in children. Clin Transl Imaging. 2016;4(1):57-72. doi:10.1007/s40336-015-0159-2
-
Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83(5):353-359. doi:/S0042-96862005000500011
-
Kay AW, Islam SM, Wendorf K, Westenhouse J, Barry PM. Interferon-? Release assay performance for tuberculosis in childhood. Pediatrics. 2018;141(6). doi:10.1542/peds.2017-3918
-
van de Vosse E, van Dissel JT. IFN-γR1 defects: Mutation update and description of the IFNGR1 variation database. Hum Mutat. 2017;38(10):1286-1296. doi:10.1002/humu.23302
-
Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15(8):968-980. doi:10.1016/S1473-3099(15)00089-4
-
van de Vosse E, van Dissel JT, Ottenhoff TH. Genetic deficiencies of innate immune signalling in human infectious disease. Lancet Infect Dis. 2009;9(11):688-698. doi:10.1016/S1473-3099(09)70255-5
-
Zimmermann P, Curtis N, Tebruegge M. Nontuberculous mycobacterial disease in childhood – update on diagnostic approaches and treatment. J Infect. 2017;74:S136-S142. doi:10.1016/S0163-4453(17)30204-9
-
Roesler J, Horwitz ME, Picard C, et al. Hematopoietic stem cell transplantation for complete IFN-γ receptor 1 deficiency: A multi-institutional survey. J Pediatr. 2004;145(6):806-812. doi:10.1016/j.jpeds.2004.08.021