Contributed by Nur-Shirin Harun, Angeline Leet, and Matthew T. Naughton , Department of Allergy, Immunology and Respiratory Medicine, The Alfred Hospital, Melbourne, Australia; Department of Cardiology, The Alfred Hospital, Melbourne, Australia; and Monash University, Melbourne, Australia
A previously well 38-year-old man was hospitalized through an Emergency Department because of progressive exertional dyspnea of 4 weeks duration accompanied by orthopnea. There was no snoring or other sign or symptoms of sleepdisordered breathing (SDB). The patient’s body mass index was 25.5 kg/m2. His heart rate was 96, and his respiratory rate was 26. Arterial oxygen saturation measured by pulse oximetry while he breathed ambient air was 95%. Physical examination revealed normal facial bony structure, clear nasal passages, and Mallampati class 1 oropharynx. Jugular venous pulsations rose to the level of his jaw when he reclined at 30 degrees elevation. Crackles were heard on auscultation over both posterior lung fields. There was an audible third heart sound, indicating a heart murmur consistent with mitral regurgitation and pedal edema. A chest radiograph showed alveolar edema and bilateral pleural effusions. An electrocardiogram displayed sinus rhythm. Left ventricular ejection fraction was estimated at 18% on a gated blood pool scan. After right heart catheterization, a diagnosis was made of idiopathic cardiomyopathy.
The patient was treated by pharmacological optimization of cardiac function and aggressive diuresis. Because of inadequate response to those measures, a left ventricular assist device (LVAD) was placed surgically on hospital Day 5 as a bridge to cardiac transplantation.
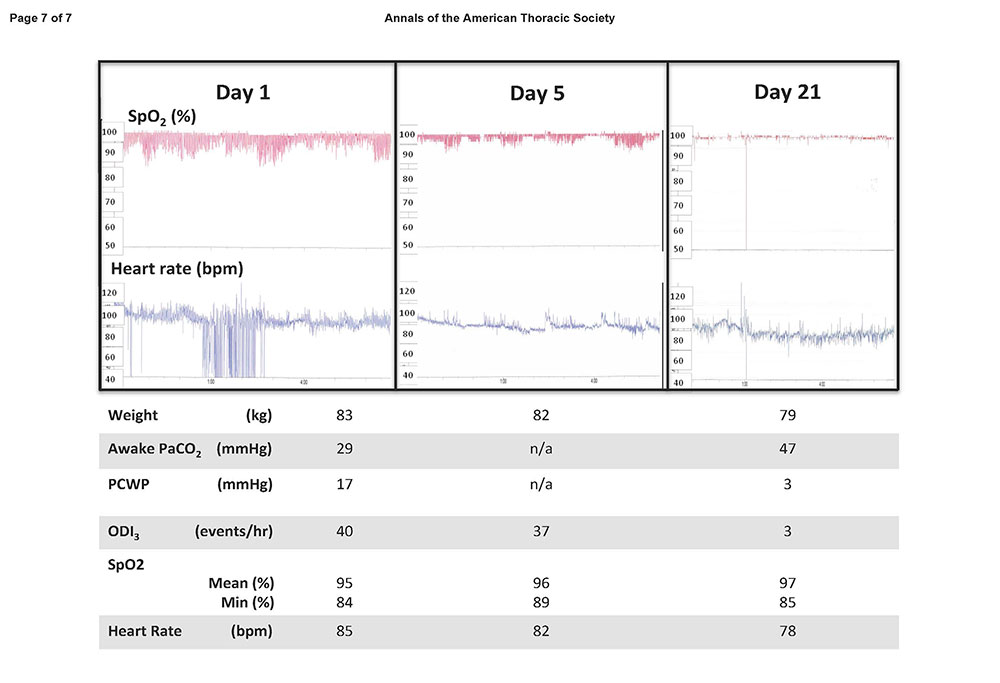
The patient’s heart rate and SpO2 were recorded continuously, and his weight, the number of times per minute that his arterial blood oxygen saturation fell 3% or more below baseline (oxygen desaturation index) (1), and his arterial blood gas tensions were measured repeatedly during his admission and are shown in Figure 1.
Figure 1. Continuous overnight oximetry recordings and other physiological data obtained on Day 1, Day 5, and Day 21 of the patient’s hospitalization. PCWP = pulmonary capillary wedge pressure; ODI3 = oxygen desaturation index of 3% or more.
Questions
What type of sleep disordered breathing was most likely before treatment?
What mechanism(s) are responsible for the change in his sleep-disordered breathing after treatment?
Discussion
Pulse oximetry on Day 1 shows a pattern consistent with severe sleep-disordered breathing. Although pulse oximetry can be sensitive and specific for moderate to severe SDB, it cannot definitively differentiate between obstructive and central sleep apnea. However, in this patient, the pattern likely represents central sleep apnea with Cheyne-Stokes respiration because of (1) maximal Sp SpO2 close to 100% throughout the night, indicating hyperventilation and low PaCO2, confirming hyperventilation; (2) frequent respiratory events occurring in the absence of severe hypoxemia; (3) rapid improvement of SDB in response to heart failure treatment (Days 1–5 = pharmacological; Days 5–21 = pharmacological with LVAD); and (4) absence of witnessed snoring.
Obstructive sleep apnea is unlikely because of the absence of snoring, a normal upper airway structure, and the prompt response to treatment for heart failure. Although rostral fluid shift may contribute to upper airway narrowing in heart failure, the effect of reversing these fluid shifts on obstructive sleep apnea severity in heart failure remains to be determined (2). Obesity hypoventilation syndrome is unlikely because of the patient’s body habitus. Chronic hypoventilation in general is excluded because of low PaCO2 and high maximal SpO2 values. Formal polysomnography with esophageal monitoring would be required to determine with certainty the type of SDB experienced by this patient before treatment.
Improved cardiac function, as indicated by a fall in pulmonary capillary wedge pressure and heart rate, probably increased our patient’s cardiac output and reduced the circulatory “feedback time” from lung to brain by PaCO2 (3). The rise in mean and minimum SpO2 values demonstrate improved pulmonary gas exchange with reduction in pulmonary edema. The rise in PaCO2 suggests reduced hyperventilation due to less circulating catecholamines and pulmonary J receptor stimulation. With fluid loss, as indicated by a fall in weight, there was probably less rostral fluid shift and thereby less pulmonary congestion and possibly less upper airway narrowing.
Although a change in sleeping posture (extra pillows or a change from supine to lateral position) could reduce central sleep apnea with Cheyne-Stokes respiration by approximately 50% (4), it is unlikely in the setting of reduced weight, complete resolution of oxygen desaturation index, and improvement in the physiological parameters listed above.
This case highlights the variability of SDB in the early stages of cardiomyopathy, responsiveness to new therapy (i.e., LVAD), and the utility of simple screening devices (overnight oximetry).
Continuous recording of pulse oximetry can be performed with equipment that is portable and easy to operate in the home and the hospital, especially in patients with severe cardiovascular instability who are too unwell to perform formal polysomnography in a sleep laboratory. However, oximetry is less accurate than polysomnography, particularly in distinguishing obstructive from central sleep apnea. Low cardiac output may impair oximetry signal quality. Moreover, certain types of LVADs provide a nonpulsatile contribution to the overall cardiac output. There may be a total loss of pulsatile flow in the periphery, thus making oximetry (and arterial blood gas sampling) difficult. On the other hand, polysomnography may be technically difficult because LVADs can provide electrical interference and difficulties with thoracic and abdominal band positioning.
Although the patient remained in sinus rhythm during the admission, Day 1 oximetry showed significant heart rate variability with mean and standard deviation of 85 6 15 beats per minute (bpm). After medical treatment and LVAD insertion, the patient’s heart rate variability normalized to 82 6 5 bpm. In our laboratory, standard deviation of heart rate is , 5 bpm in patients with no or mild SDB and , 10 bpm in patients with moderate SDB.
Answer
Likely sleep disorder diagnosis: central sleep apnea with Cheyne-Stokes respiration.
Mechanisms for improvement after treatment: increased cardiac output, reduced pulmonary edema, and reduced sympathetic activity.
Follow-Up
Six months after placement of the LVAD, the patient underwent orthotropic cardiac transplantation, which was complicated subsequently by acute humoral rejection. He remains asymptomatic from the sleepdisordered breathing perspective and is yet to undergo formal polysomnography given his current medical issues.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
-
Seri´ es` F, Kimoff RJ, Morrison D, Leblanc MH, Smilovitch M, Howlett J, Logan AG, Floras JS, Bradley TD. Prospective evaluation of nocturnal oximetry for detection of sleep-related breathing disturbances in patients with chronic heart failure. Chest 2005;127:1507–1514.
-
Redolfi S, Arnulf I, Pottier M, Lajou J, Koskas I, Bradley TD, Similowski T. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med 2011;184:1062–1066.
-
Naughton MT, Andreas S. Sleep apnoea in chronic heart failure. Eur Respir Mon 2010;50:396–420.
-
Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position promotes ventilatory stability in central sleep apnea / Cheyne-Stokes respiration. Sleep 2006;29:1045– 1051.
Harun N, Leet A, and Naughton MT. Improvement in sleep-disordered breathing after insertion of left ventricular assist device. Ann Am Thorac Soc 2013;10:272-273.