Contributed by Sandeep Khosa1, Mazen El Ali2, and Vidya Krishnan1 1Department of Pulmonary, Critical Care, and Sleep Medicine, MetroHealth Medical Center, Cleveland, Ohio; and 2Department of Pulmonary, Critical Care, and Sleep Medicine, Case Western Reserve University, Cleveland, Ohio
In Brief
One of the challenges in interpreting sleep studies is determining whether findings are real or artifacts of electromechanical phenomena. The artifact seen on this study was consistent with the patient’s history, but was an unusual finding for typical patients undergoing sleep studies. The findings are reviewed in the context of the clinical presentation of the case patient.
Case Vignette
A 24-year-old man presented for evaluation of continued need for cannulation of his tracheostomy after successful liberation from mechanical ventilation. He had sustained a C3–C4 spinal cord injury (SCI) 3 years previously, resulting in C3 ASIA (American Spinal Injury Association) A tetraplegia. After tracheostomy and prolonged dependence on mechanical ventilation, a procedure was performed to facilitate liberation from the ventilator.
The patient endorsed no symptoms of fatigue or sleepiness (Epworth Sleepiness Scale score, 2/24). On physical examination, his body mass index was 20.85 kg/m2. Slight nasal septum deviation to the left and elongated uvula were noted. Flaccid paralysis in all extremities was observed, with 0/5 strength and no sensation. He had a size 4 cuffless Shiley tracheostomy tube. A tracheobronchoscopy performed 6 months previously revealed no endotracheal granulation tissue or stenosis.
Diagnostic, in-laboratory nocturnal polysomnograms with transcutaneous CO2 (PtcCO2) monitoring were performed and representative sleep fragments are shown. Figures 1 and 2 are epochs with the tracheostomy open, during wakefulness, and during stage N3 sleep, respectively. Figure 3 is an epoch with capping of the tracheostomy during stage N3. When the tracheostomy was open, the pressure transducer was placed at the tracheostomy and the thermistor at the patient’s nose and mouth. When the tracheostomy was closed, both airflow sensors were placed at the patient’s nose and mouth. PtcCO2 signals could not be integrated in the polysomnogram software, and therefore were recorded intermittently throughout the study by the recording sleep technician.
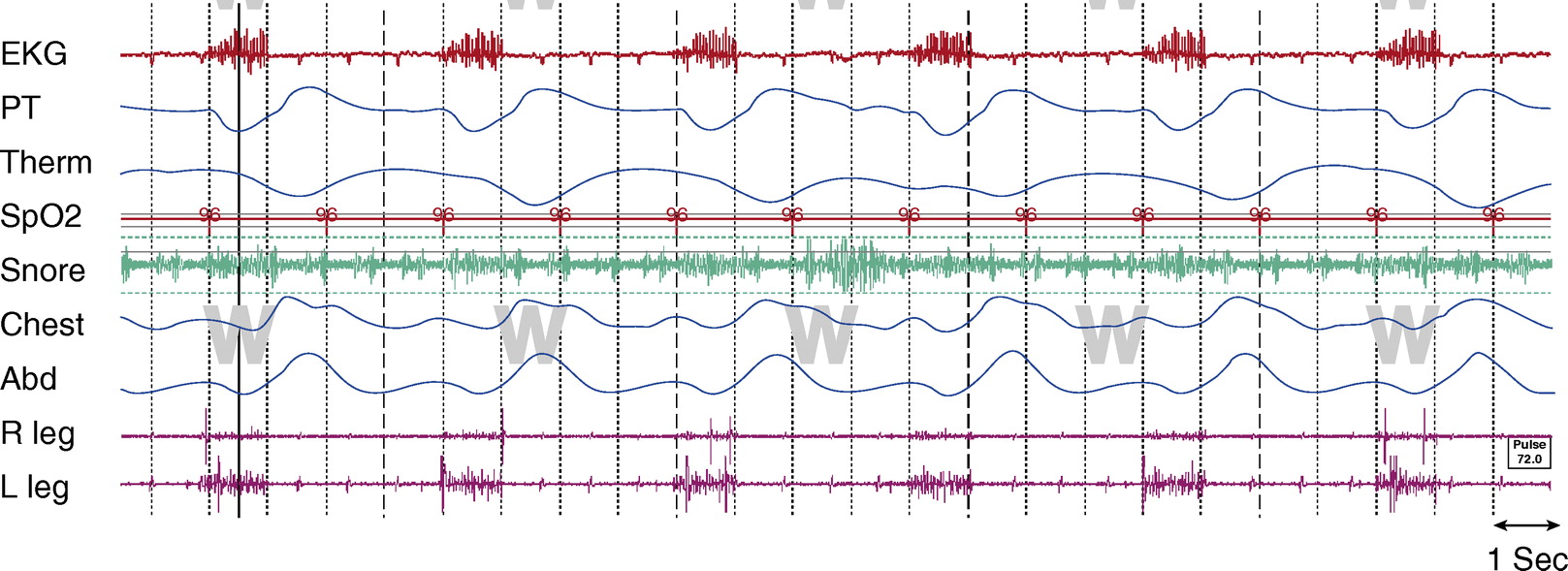
Figure 1.
Awake, tracheostomy open. TcCO2 was 40 to 45 mm Hg throughout study. Chest and Abd = piezo-electric respiratory belts for chest and abdomen, respectively; EKG = electrocardiogram; PT = pressure transducer; R leg and L leg = electromyogram of right leg and left leg, respectively; SpO2 = pulse oximetry–determined oxygen saturation; Tcco2 = transcutaneous CO2; Therm = thermistor.
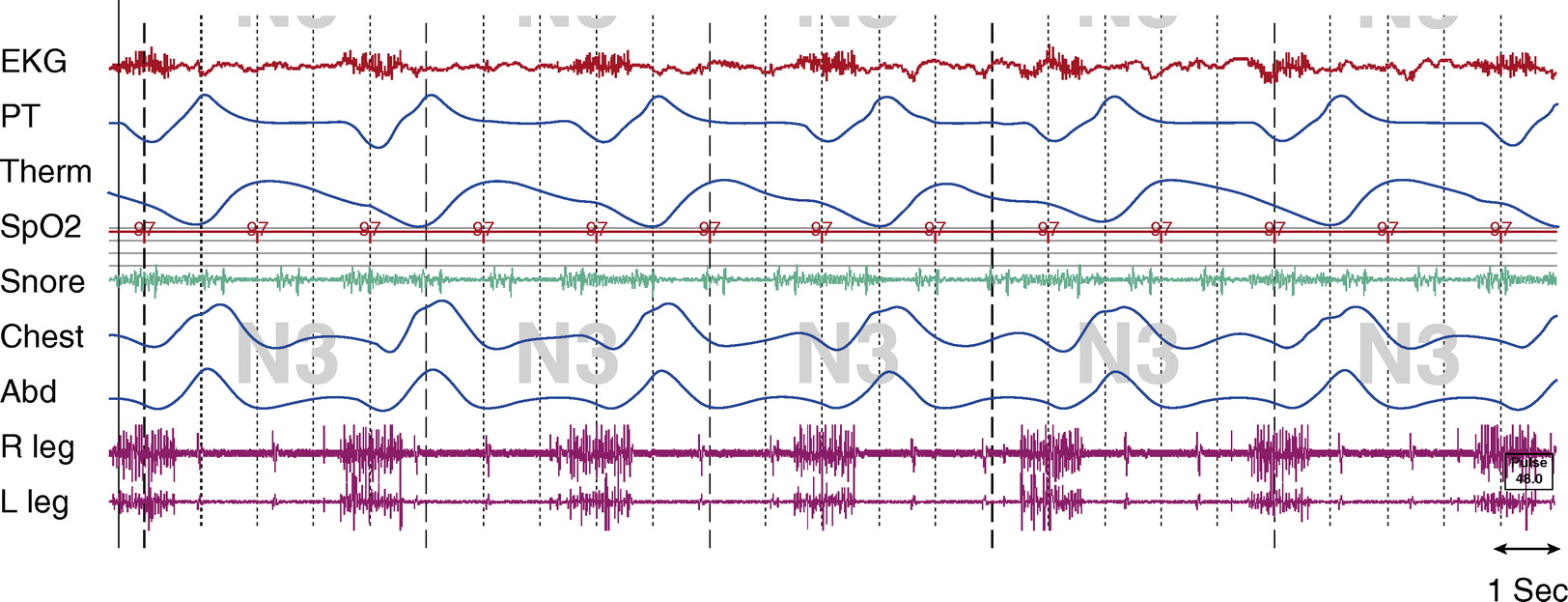
Figure 2.
Stage N3, tracheostomy open.Tcco2 was 40 to 45 mm Hg throughout study. Chest and Abd = piezo-electric respiratory belts for chest and abdomen, respectively; EKG = electrocardiogram; PT = pressure transducer; R leg and L leg = electromyogram of right leg and left leg, respectively; Spo2 = pulse oximetry–determined oxygen saturation; Tcco2 = transcutaneous CO2; Therm = thermistor.
Questions
1. What is the artifact that persists throughout the sleep fragments?
2. Is there evidence of airflow obstruction during sleep when the tracheostomy was closed?
Discussion
Signal artifacts should be considered when a signal pattern is (1) found in multiple channels, (2) observed at regular time intervals, (3) constant in duration when intermittent, and/or (4) synchronized with other physiologic cycles (e.g., respiratory cycle, cardiac cycle). Additional signals may also help clarify the source of the artifact. In this case, the DPS signal was found at regular intervals, for a fixed duration, and coincided with the inspiratory limb of the respiratory cycle. Additional EMG signals placed near the midaxillary line and midthorax may have detected a high-intensity signal originating from the DPS.
For patients dependent on mechanical ventilation, there are two major techniques for the conversion of mechanical to electrical ventilation: phrenic nerve stimulation (PNS) and DPS. Because the PNS requires intact bilateral phrenic nerve function, most patients with cervical SCI are not candidates for this procedure (1). An attractive option is the DPS, as is present in our patient. They have been shown to reduce infections and nursing needs, while improving patient mobility, speech, and overall quality of life (1, 2).
The DPS has several advantages. The diaphragm electrodes can be implanted laparoscopically, thereby avoiding the need for thoracotomy. Phrenic nerve dissection is not required and minimizes potential nerve injury (1). In a large worldwide study, DPS has been shown to delay, and even eliminate, the need for mechanical ventilation in a select group of patients with SCI. In patients with SCI who underwent DPS implantation, 96% were successfully liberated from long-term mechanical ventilation (3).
SCI is associated with a 2- to 5-fold higher prevalence of sleep-disordered breathing than the general population. One study has reported the prevalence of sleep apnea (apnea–hypopnea index [AHI] . 5) among SCI survivors with cervical or high thoracic injuries to be 77%, and 92% reported poor sleep quality (4). Patients with SCI are at risk for sleep-disordered breathing for a number of reasons. Patients with SCI are more commonly male, are often obese due to inactivity, and are frequently positioned in the supine position—three risk factors for obstructive sleep apnea. Weakness of the diaphragm and other respiratory muscles contributes to hypoventilation. In addition, the lower lung volumes due to muscular weakness reduce the natural tracheal tug that contributes to upper airway patency. Patients with SCI also have altered respiratory mechanics due to paradoxical rib cage and abdomen movement during inspiration. Finally, muscle relaxant medications prescribed for patients with SCI can contribute to airway muscle relaxation, promoting upper airway collapse.
The effects of electrical stimulation of breathing on sleep are uncertain. DPS has been successfully used to treat congenital central hypoventilation syndrome (5). In patients with amyotrophic lateral sclerosis, improved sleep is reported post-DPS placement, attributed to improved diaphragm function and strengthening (6). Conversely, new-onset obstructive sleep apnea has been reported after diaphragm pacing in patients with primary alveolar hypoventilation syndrome (7, 8). This is thought to be due to the imbalance of activity between the upper airway dilator muscles and the respiratory pump muscles. With increasing use of these devices, further research on effects of diaphragm pacing on sleep is needed.
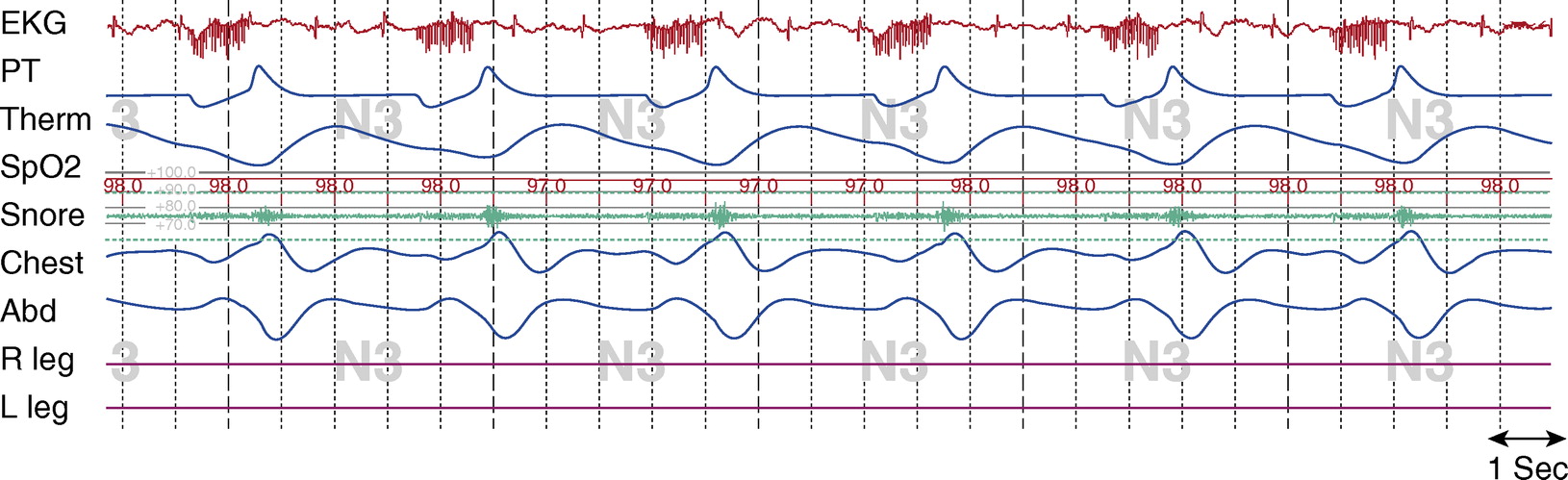
Figure 3. Stage N3, tracheostomy closed. Tcco2 was 40 to 43 mm Hg at beginning of study; total sleep time with Tcco2 > 50 mm Hg, about 15%; maximum Tcco2 recorded, 53 mm Hg. Chest and Abd = piezo-electric respiratory belts for chest and abdomen, respectively; EKG = electrocardiogram; PT = pressure transducer; R leg and L leg = electromyogram of right leg and left leg, respectively; Spo2 = pulse oximetry–determined oxygen saturation; Tcco2 = transcutaneous CO2; Therm = thermistor. Note: R and L leg EMG signals malfunctioned during this study.
Answers
-
What is the artifact that persists throughout the sleep fragments?
The artifact is generated from an implanted diaphragm pacing stimulator (DPS). The artifact is most prominent in the ECG and EMG signals, as seen in Figures 1 and 2. The duration of the electrical stimulation is precisely the duration of inspiration, and these intermittent electric impulses occur every 4 seconds (DPS set at 15 breaths/min) consistently across sleep and wakefulness.
-
Is there evidence of airflow obstruction during sleep when the tracheostomy was closed?
There is evidence of flow limitation when the tracheostomy is capped. In Figure 3, there is evidence of snoring, which occurs after the DPS signal during expiration. There is flattening of the inspiratory limb of the airflow channel and reductions of airflow. Paradoxical chest and abdomen efforts may be a result of the flow limitation, or may be due to quadriplegia and subsequent abnormal respiratory mechanics.
Follow-Up
With the tracheostomy open, there was no evidence of flow limitation, the total AHI was 0, the oxyhemoglobin saturation (SpO2) remained above 95%, and CO2 levels were normal. With the tracheostomy capped, however, while there was no evidence of airflow limitation during wakefulness (data not shown), there was during sleep. The AHI was 10.1 events/hour, the lowest SpO2 was 90%, and the CO2 levels rose to a maximum of 53 mm Hg. Given the continued flow limitation with the tracheostomy capped, residual mild obstructive sleep apnea, and nocturnal hypoventilation, we did not recommend decannulation of the tracheostomy without additional treatment of obstructive sleep apnea.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
-
DiMarco AF. Neural prostheses in the respiratory system. J Rehabil Res Dev 2001;38:601–607.
-
Lewis MI. Diaphragm disorders: paralysis, hernia, eventration. In: Lewis MI, McKenna RJ, Jr, editors. Medical management of the thoracic surgery patient, 1st ed. Philadelphia, PA: Saunders Elsevier; 2010, pp. 468–476.
-
Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, Bass B, Dunkin B, Ingvarsson PE, Oddsdo´ ttir M. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc 2009;23:1433–1440.
-
Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med 2014;10:65–72.
-
Chen ML, Tablizo MA, Kun S, Keens TG. Diaphragm pacers as a treatment for congenital central hypoventilation syndrome. Expert Rev Med Devices 2005;2:577–585.
-
Gonzalez-Bermejo J, More´ lot-Panzini C, Salachas F, Redolfi S, Straus C, Becquemin MH, Arnulf I, Pradat PF, Bruneteau G, Ignagni AR, et al. Diaphragm pacing improves sleep in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2012;13:44–54.
-
Moue Y, Kamio K, Tanigaki T, Hayashi Y, Kuwahira I, Takasaki Y, Ohta Y, Yamabayashi H. [Successful treatment of diaphragm pacing-induced obstructive sleep apnea syndrome with nasal CPAP] [in Japanese]. Nihon Kyobu Shikkan Gakkai Zasshi 1993;31:990–993.
-
Hyland RH, Hutcheon MA, Perl A, Bowes G, Anthonisen NR, Zamel N, Phillipson EA. Upper airway occlusion induced by diaphragm pacing for primary alveolar hypoventilation: implications for the pathogenesis of obstructive sleep apnea. Am Rev Respir Dis 1981;124:180–185.