Contributed by Corresponding Author: Gaurav Nigam, MD University of Michigan Sleep Disorders Center C728 Med Inn Bldg. | 1500 E. Medical Center Drive Ann Arbor, MI 48109-5845 Phone: 612.916.2993 | dr.nigamgaurav@gmail.com Disclosure Statement: Authors do not have anything to disclose, this study was not supported by industry. The views expressed herein are solely of the authors. Conflict of Interest: None Acknowledgement: None Keywords: REM rebound, continuous positive airway pressure, cortical arousal, adherence, obstructive sleep apnea.
In Brief
A 64-year-old man was evaluated for symptoms suggestive of severe obstructive sleep apnea. A split-night sleep study confirmed this diagnosis. At the onset of CPAP titration, a polysomnographic phenomenon occurred that may have clinical implications for patients with obstructive sleep apnea.
Case Vignette
64-year-old man presented with complaints of snoring, witnessed apneas (as noted by his bed partner) and excessive daytime sleepiness ongoing for about 10 years prior to presentation. He had hypertension, atrial fibrillation, 3-vessel coronary artery disease and congestive heart failure. He had been smoking cigarettes (0.5 packs per day) for more than 25 years. The man denied use of alcohol or recreational drugs. His medications included atorvastatin, amiodarone, chlorthalidone, furosemide, metoprolol, glipizide, metformin, warfarin and gabapentin. His body mass index (BMI) was 32 kg/m2. Physical examination was otherwise unremarkable.
Patient underwent an in-laboratory split-night polysomnographic study (PSG) that demonstrated severe obstructive sleep apnea (OSA) with an apnea-hypopnea index (AHI) of 93.5 events/hour and an oxygen saturation nadir of 73%. No REM sleep was achieved during the baseline portion of the study. Given the severity of OSA on the baseline portion of the study, continuous positive airway pressure ventilation (CPAP) was subsequently initiated. At a pressure of 20 cm H2O, CPAP effectively abolished sleep apneas. Bi-level PAP was not required and hence not tested.
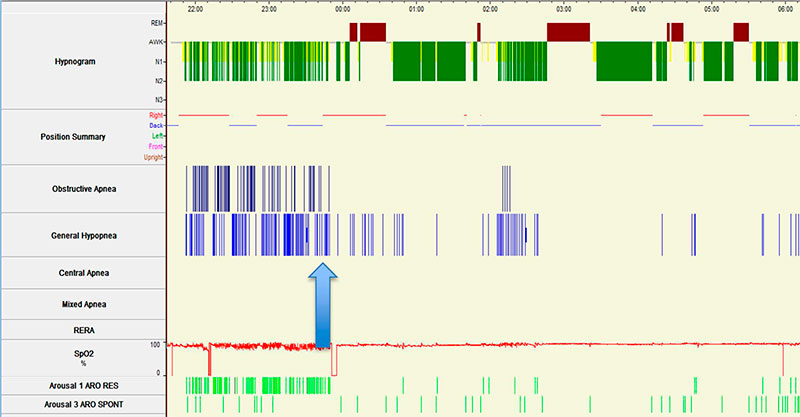
Figure 1. Split-night sleep study summary of the hypnogram, respiratory events and body position*.
Arrow indicates point of initiation of CPAP during the split-night study
*Sleep position:
Blue horizontal line: Supine position
Red horizontal line: Right lateral position
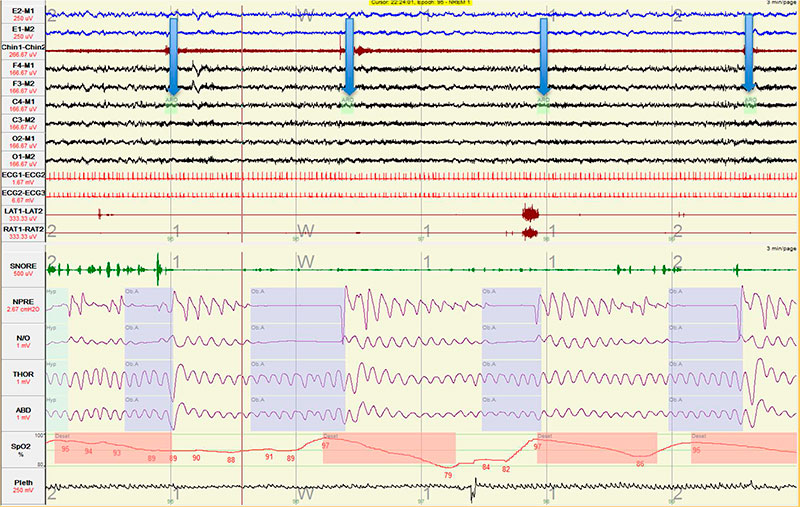
Figure 2. Multiple obstructive apneas in fast succession resulting in sleep fragmentation during the diagnostic portion of the split-night sleep study.
The split-night study hypnogram and respiratory events are shown in Figure 1. As shown in Figure 2, sleep architecture was marked by multiple obstructive apneas leading to arousals (indicated by downward pointing arrows).
Questions
-
In the hypnogram for this patient (Figure 1), what polysomnographic phenomenon occurred on first exposure to CPAP?
-
What impedes attaining and/or maintaining REM sleep in patients with severe untreated OSA, as demonstrated in Figure 2?
-
What are the potential clinical implications of this phenomenon?
Discussion
The hypnogram for this patient (Figure 1) demonstrates REM rebound on the first night of exposure to CPAP. REM rebound on CPAP is a polysomnographic phenomenon that refers to an increased percentage of REM sleep on first exposure to CPAP as compared to baseline. Although formal consensus is lacking, an increase of 20 % or more in the percentage of REM sleep during the titration phase compared to the baseline phase of a split-night sleep study is widely considered indicative of REM rebound (1). Similarly, when diagnosis and titration occur on two separate nights, an increase of 6 to 20% or more in the percentage of REM sleep on the titration as compared to baseline polysomnogram is indicative of REM rebound (2-3).
Other changes can occur on first exposure to CPAP. In addition to REM rebound there can be an increase in slow wave sleep (or stage N3 rebound) (1). Also in untreated OSA, a concurrent decrease in the percentages of stages N1 and N2 sleep can occur on first exposure to CPAP during full-night titration studies (2).
Several factors predispose to REM suppression and predict rebound on first exposure to CPAP. In patients with untreated OSA, cortical arousals that are observed near the end of the majority of obstructive events may facilitate restitution of airway patency. In the case of stage REM sleep, the balance between arousal threshold and upper airway opening threshold is tipped in favor of earlier arousal (4). This high frequency of arousals, each triggered by an individual obstructive apnea or hypopnea, prevents attaining or maintaining REM sleep.
Early experimental studies of selective sleep deprivation demonstrated that the number of “arousals containing epochs” required to abort ongoing REM sleep is 4 times lower than the number of “arousals containing epochs” required to disrupt slow-wave sleep, corroborating that REM sleep is highly susceptible to eradication by arousals (5). Muscle atonia, a characteristic of REM sleep, encompasses inhibition of upper airway and intercostal muscle activation even in the later apneic phase (6). This could result in profound oxygen desaturation rand hypoventilation during protracted episodes of obstructive apnea. Hence delaying or abbreviating REM sleep period may be an evolutionary protective mechanism to avoid apnea and hypoventilation in a patient with untreated OSA.
Consequently, several OSA-related factors could predispose to REM rebound. A high baseline AHI is a well-established predictor (1); also, a low percentage of REM sleep on a full night baseline PSG due to untreated OSA could lead to REM rebound on the subsequent full night titration study (2, 3). Non-OSA related factors that could result in REM rebound in patients with OSA include acute sleep deprivation and withdrawal of REM-suppressants (MAOIs, SSRIs and SNRIs and alcohol).
A polysomnographic rebound in stage-REM sleep following first exposure to CPAP has been correlated with subjective improvement in sleep quality (7). Some authors have speculated that REM rebound can be useful as a prognostic indicator for early CPAP adherence (2, 7). One study did show that REM rebound on the first night of exposure to CPAP was associated with improved CPAP adherence at 30 and 60 days, but not at 120 days (1). More studies will be needed to consolidate these findings, clarify the predictive role in long-term CPAP adherence, and to understand if percentage of REM rebound is directly proportional to the magnitude of CPAP adherence. Improvement in sleep efficiency from diagnostic to the titration night and consequent reduction in excessive daytime sleepiness could be other factors predictive of adherence to CPAP (8).
Answers
-
The hypnogram demonstrates REM rebound on first night of exposure to CPAP.
-
Frequent and repetitive cortical arousals associated with obstructive apneas and hypopneas impede attaining/maintaining REM, given REM compared to NREM sleep is swiftly dissipated by the recurrent arousals.
-
Some evidence suggests that REM rebound on first exposure to CPAP can predict improved early CPAP adherence, although this improved adherence may not persist long-term.
Follow-Up
At a 3-month follow-up clinic visit, adherence summary revealed that CPAP at 20 cmH2O was used on 81 of the first 90 nights. CPAP device was used on an average for 6 hours and 5 minutes every night over the 90 day period and for 6 hours and 45 minutes on nights in which CPAP was used. The patient used CPAP for longer than 4 hours/night on 83% of the nights. Residual AHI was within acceptable limits at 4.2 events/hour. Thus, our patient had an excellent CPAP adherence. He reported substantial resolution of excessive daytime sleepiness, and no recurrence of snoring or reported witnessed apneic events.
References
-
Koo BB, Wiggins R, Molina C. REM rebound and CPAP compliance. Sleep Med. 2012 Aug;13(7):864-8.
-
Brillante R, Cossa G, Liu PY, Laks L. Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea. Respirology. 2012 Apr;17(3):547-53.
-
Osuna E, Siddiqui F, Walters A, Chokroverty S. Prevalence and factors affecting REM rebound on CPAP titration study in patients with obstructive sleep apnea. Sleep Abstract Supplement; June 9-14, 2007; Minneapolis. ; 2007.
-
Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004 Mar 1;169(5):623-33.
-
Agnew HW,Jr, Webb WB, Williams RL. Comparison of stage four and 1-rem sleep deprivation. Percept Mot Skills. 1967 Jun;24(3):851-8.
-
Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994 Sep;106(3):767-73.
-
Verma A, Radtke RA, VanLandingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001 May;2(3):215-23.
-
Drake CL, Day R, Hudgel D, Stefadu Y, Parks M, Syron ML, Roth T. Sleep during titration predicts continuous positive airway pressure compliance. SLEEP. 2003 26(3), 308-312.



